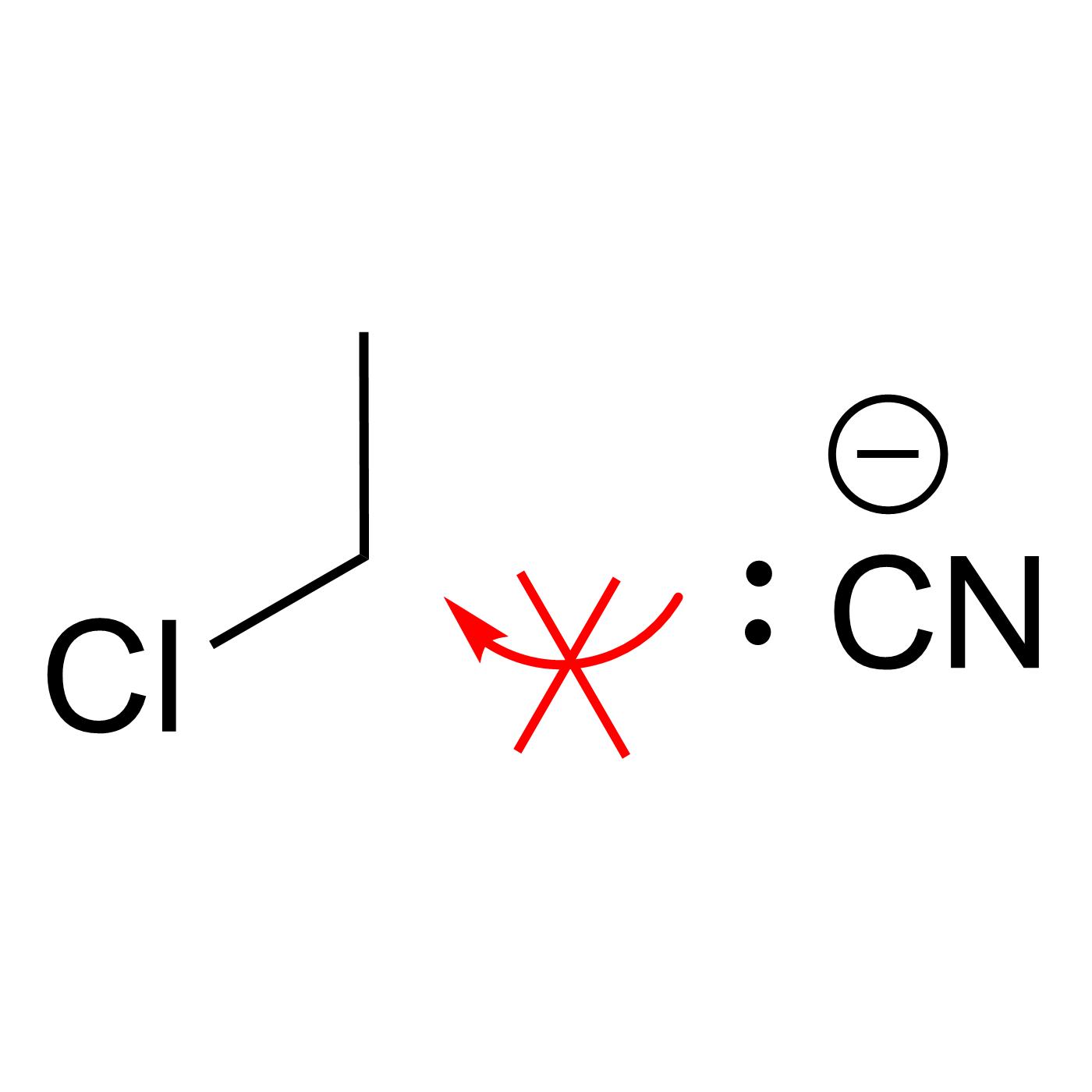
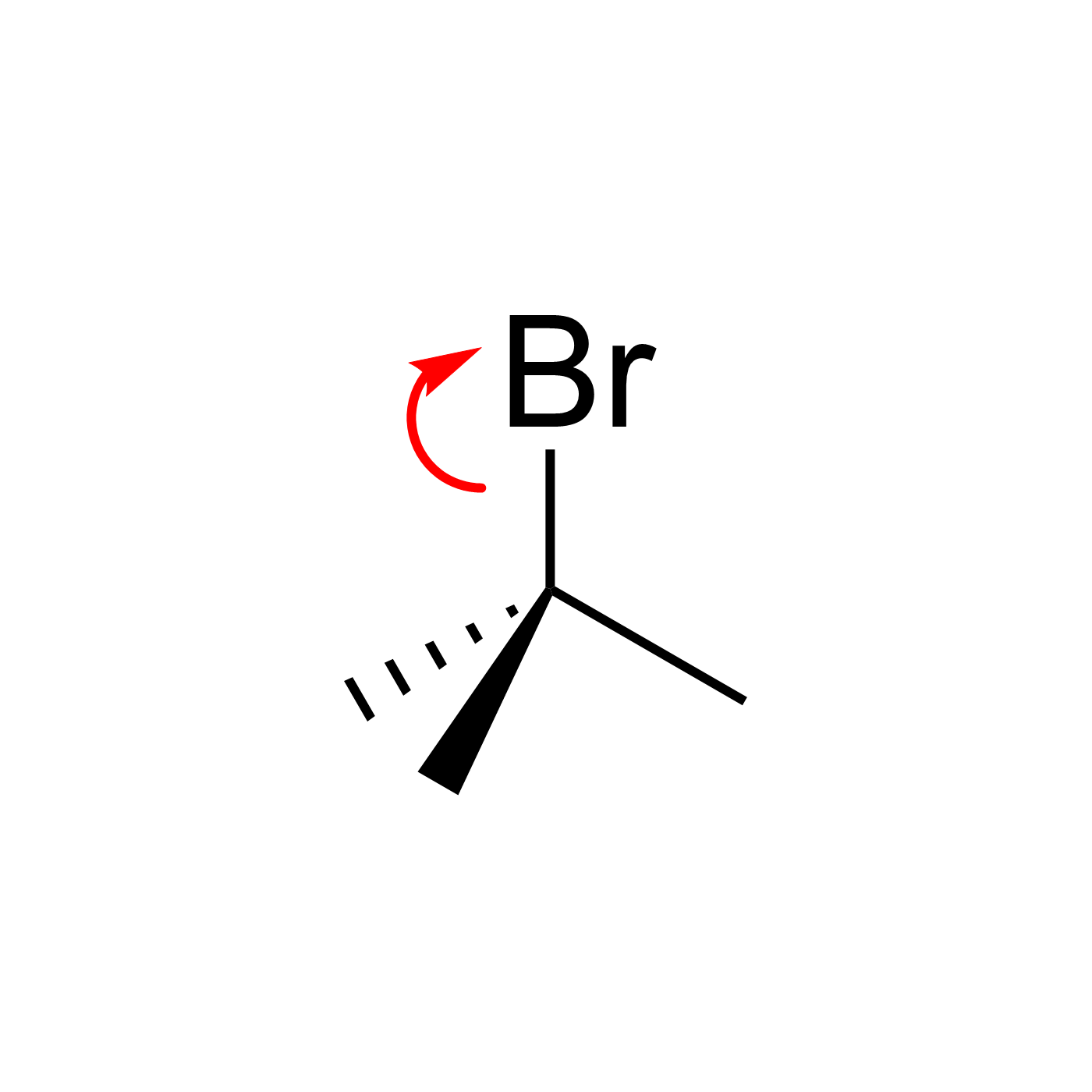
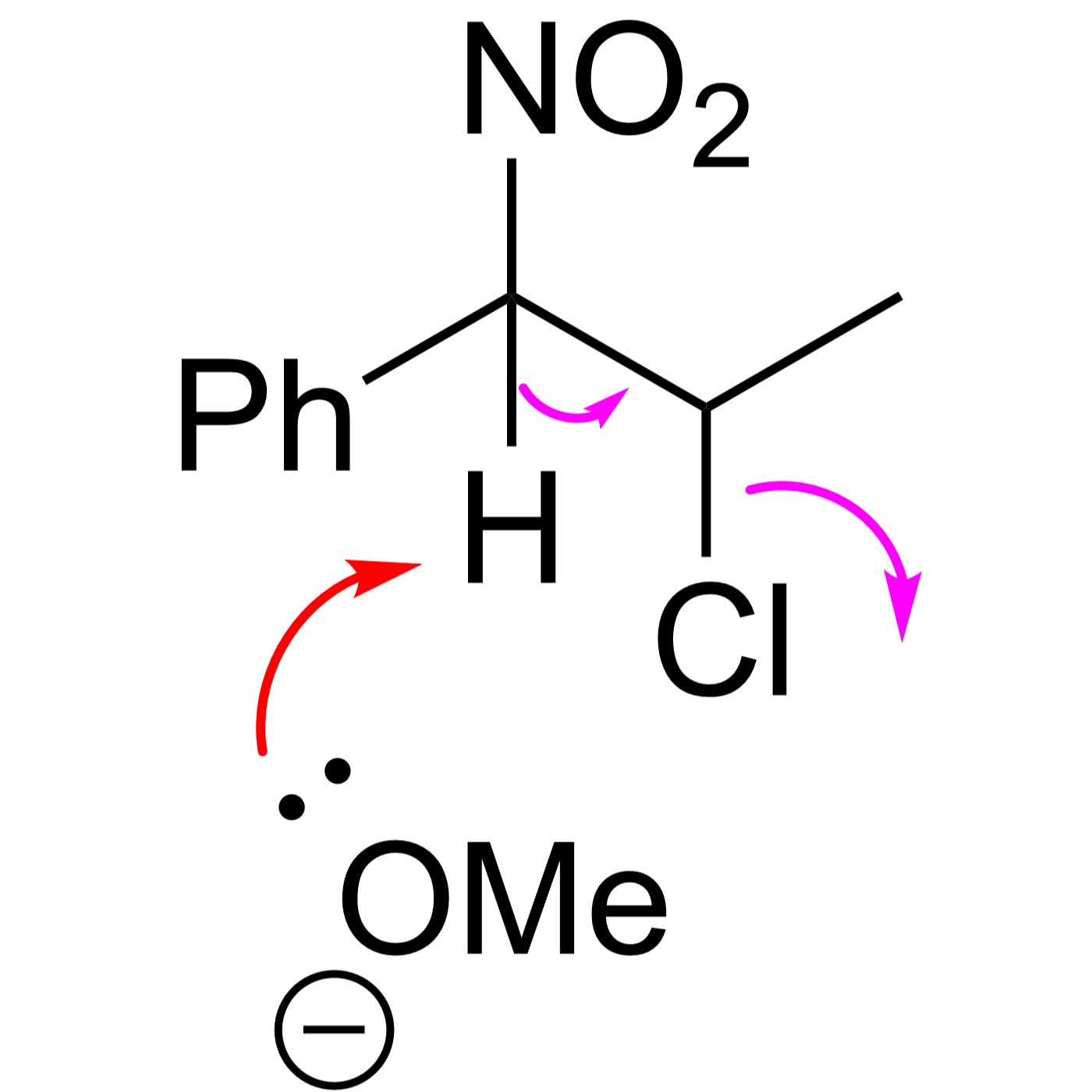
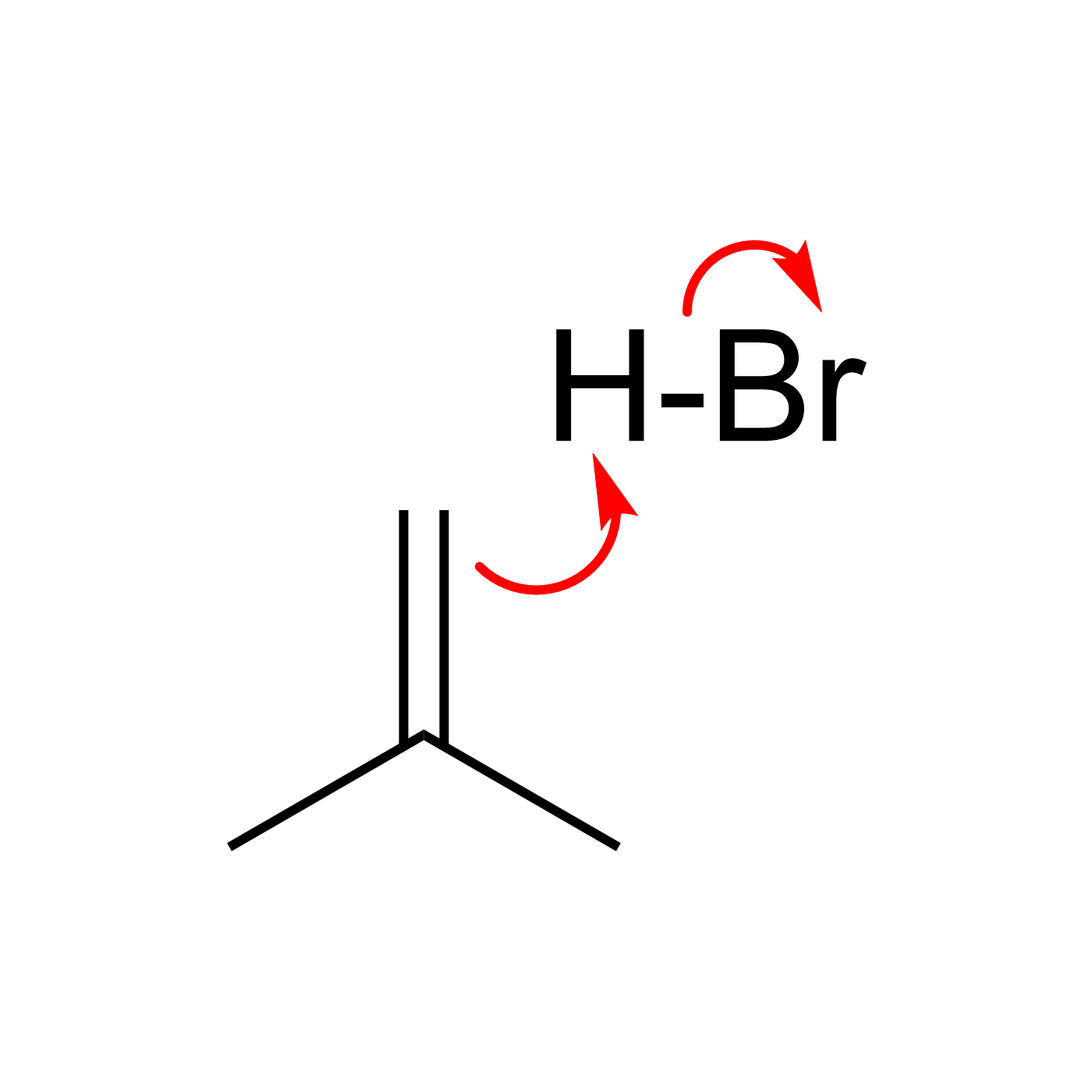
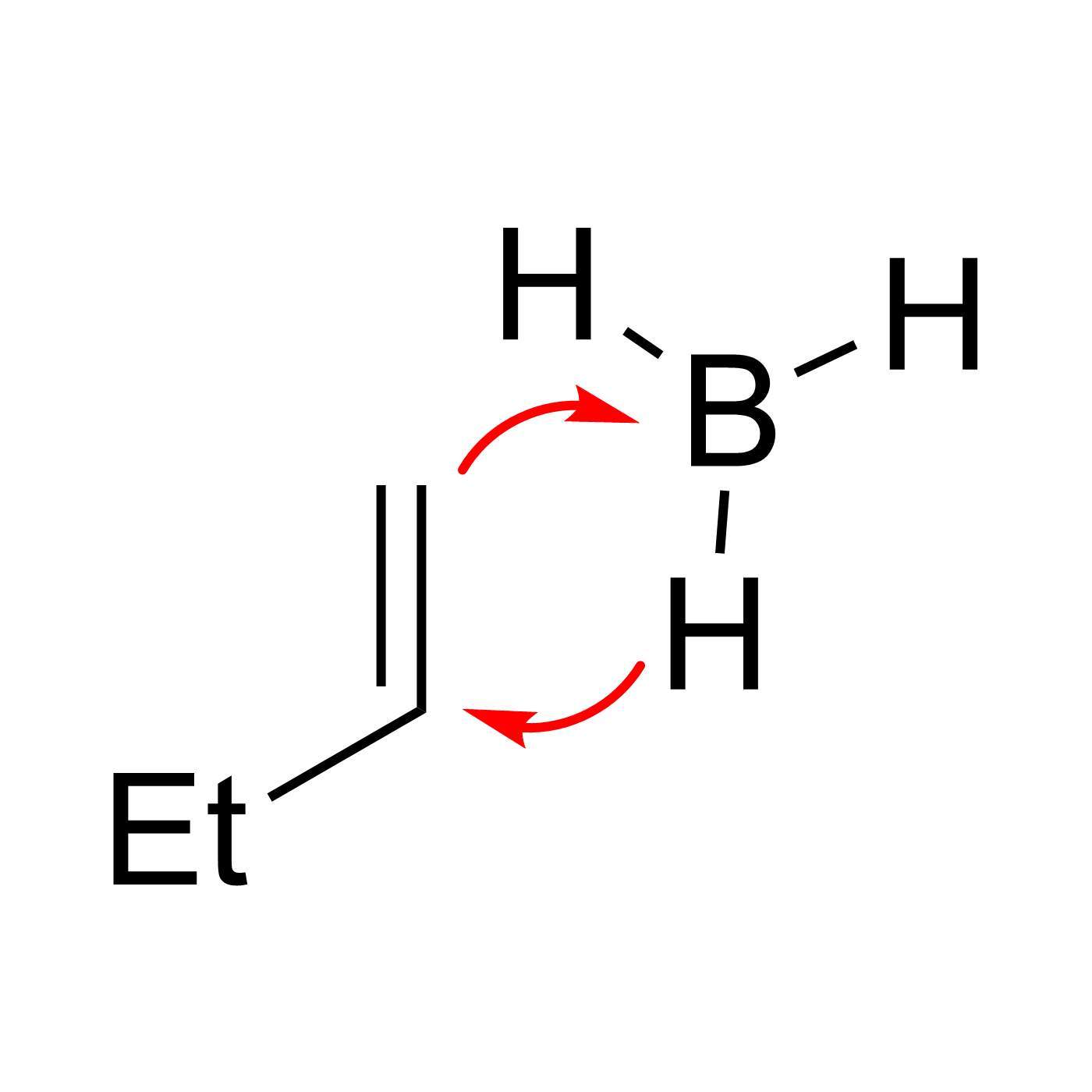
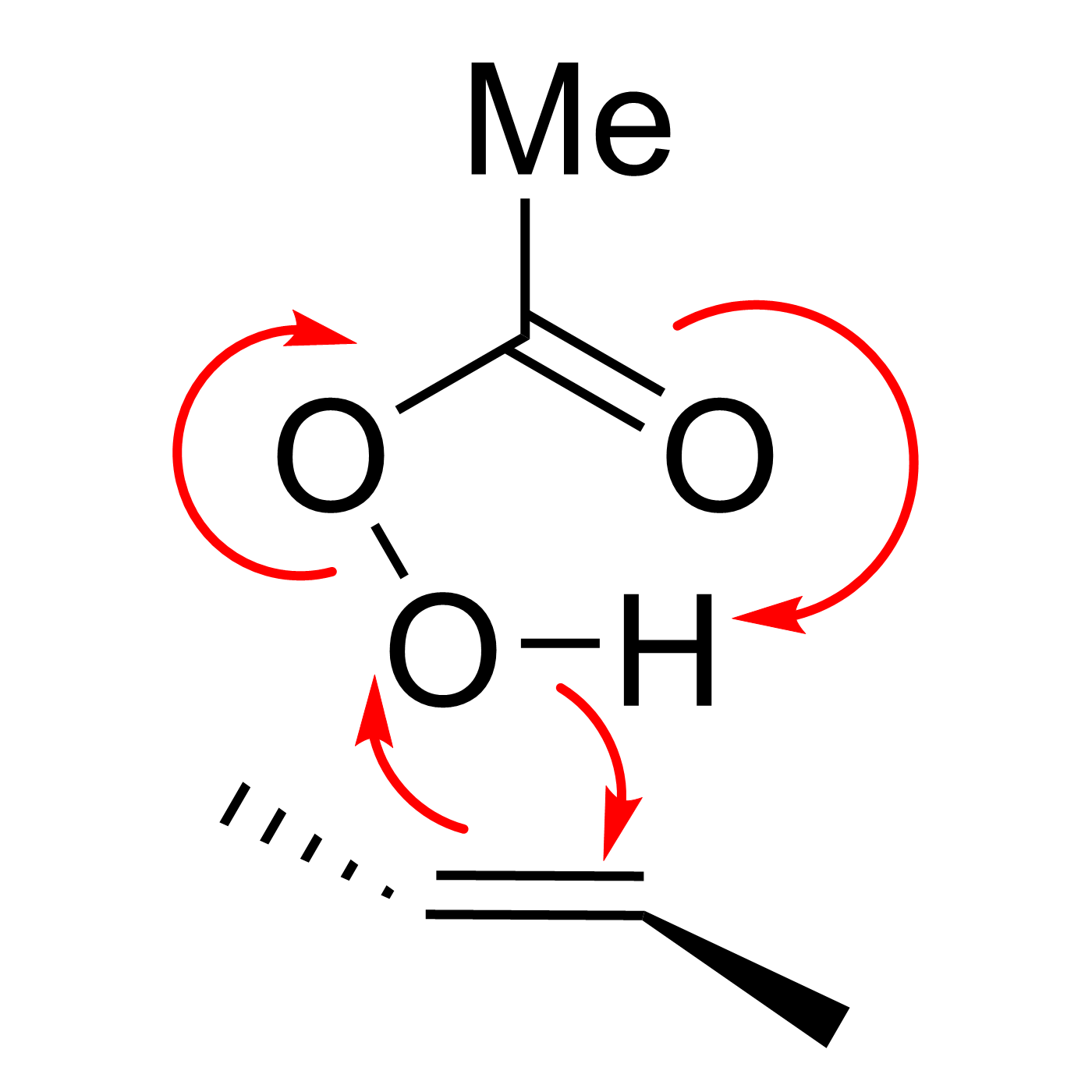
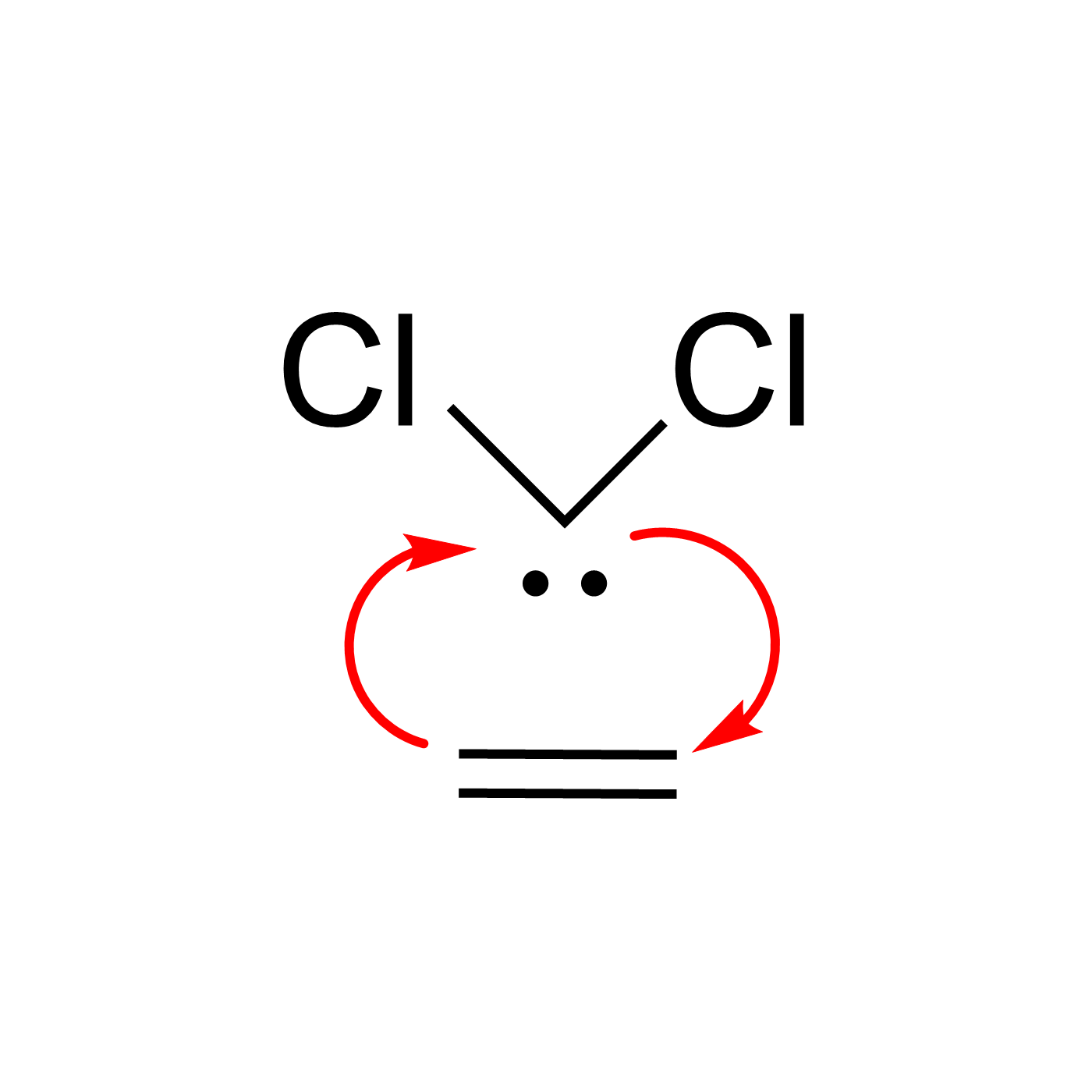
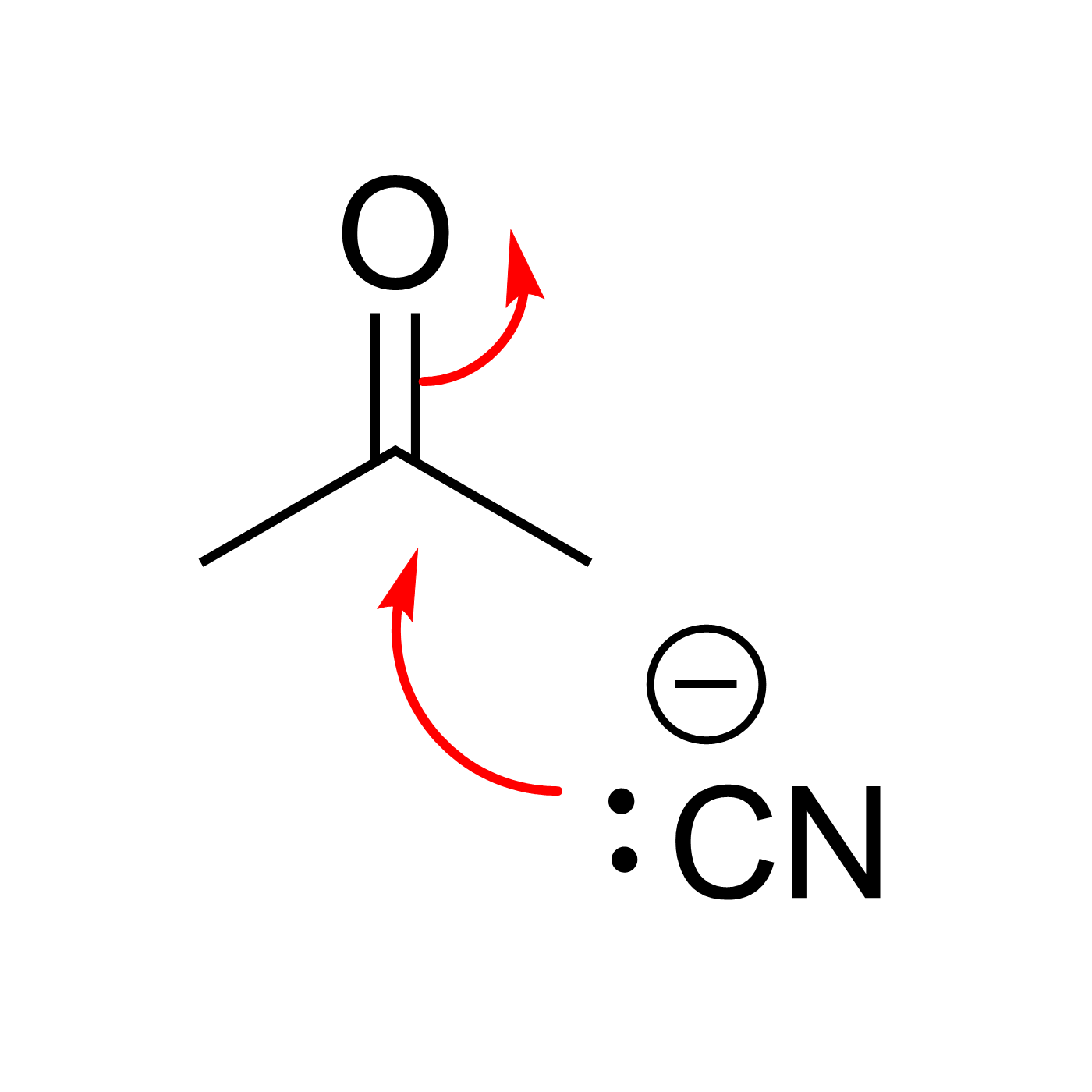
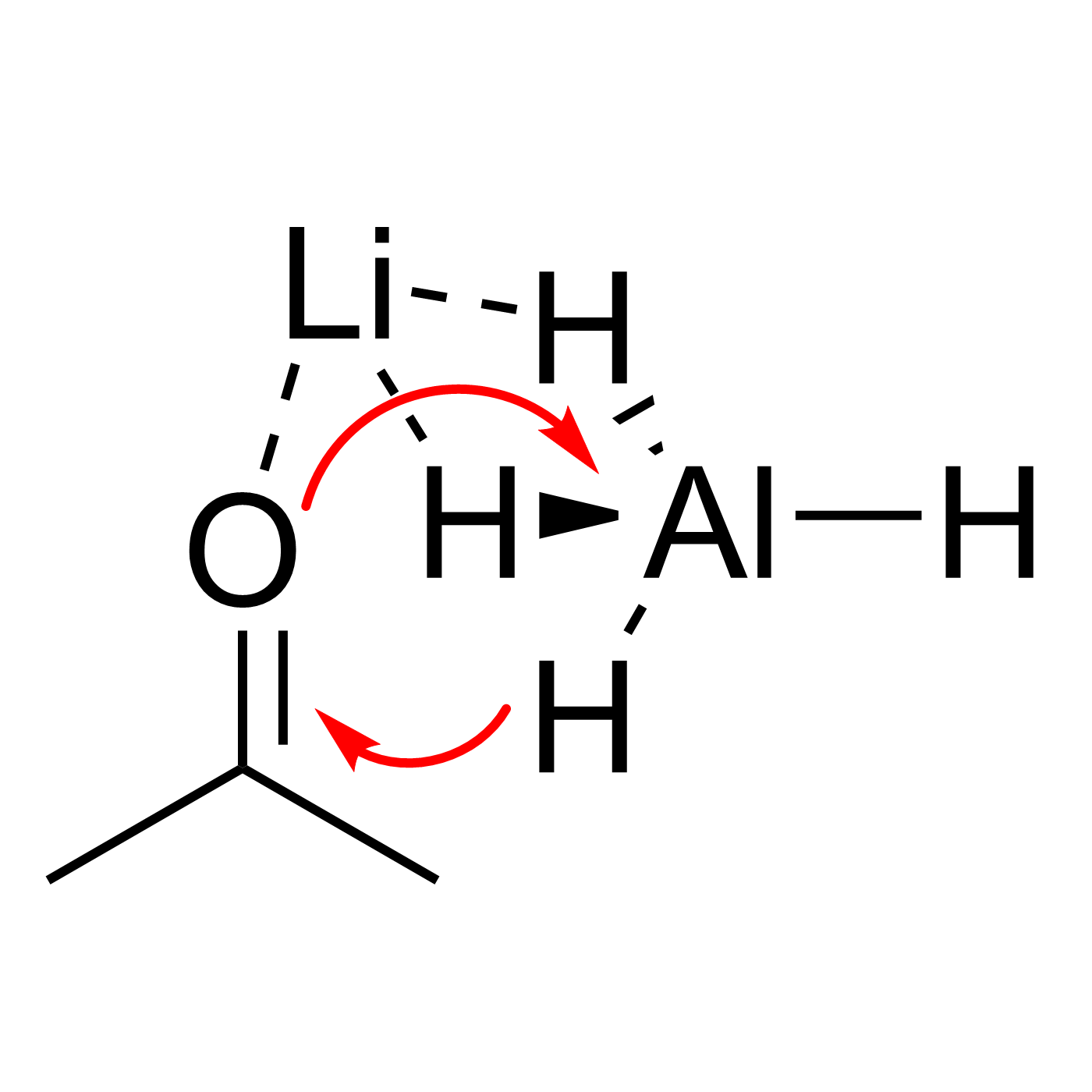
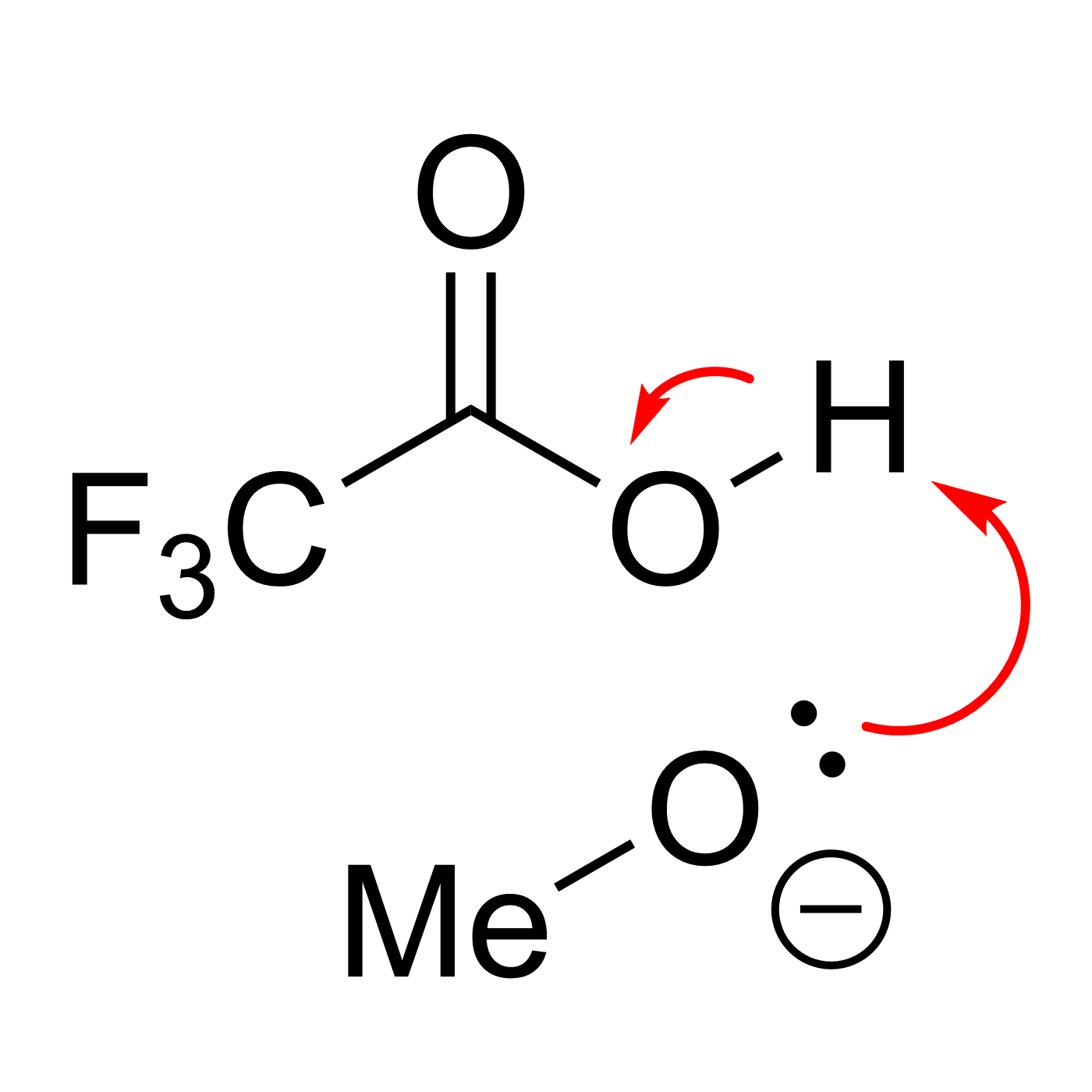
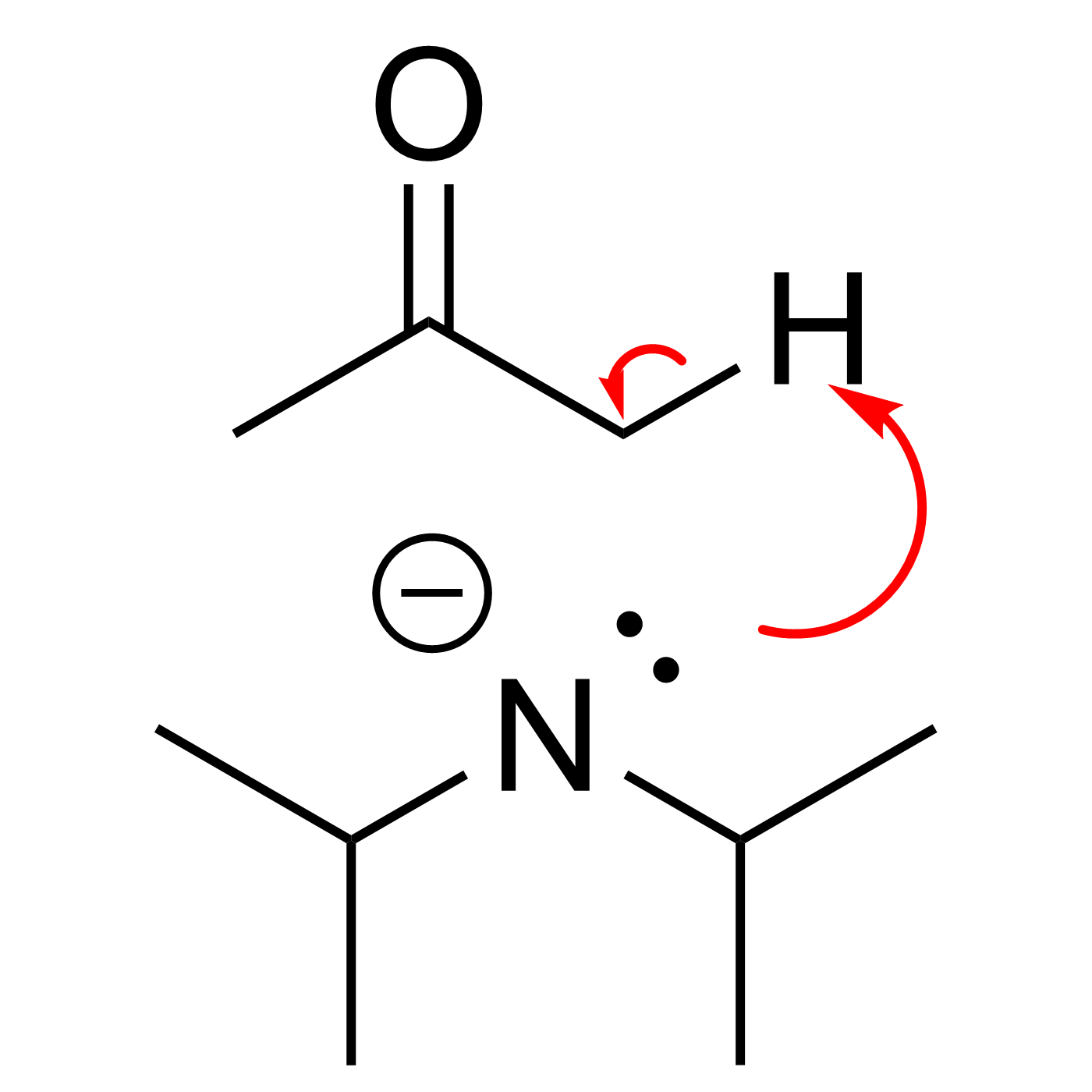
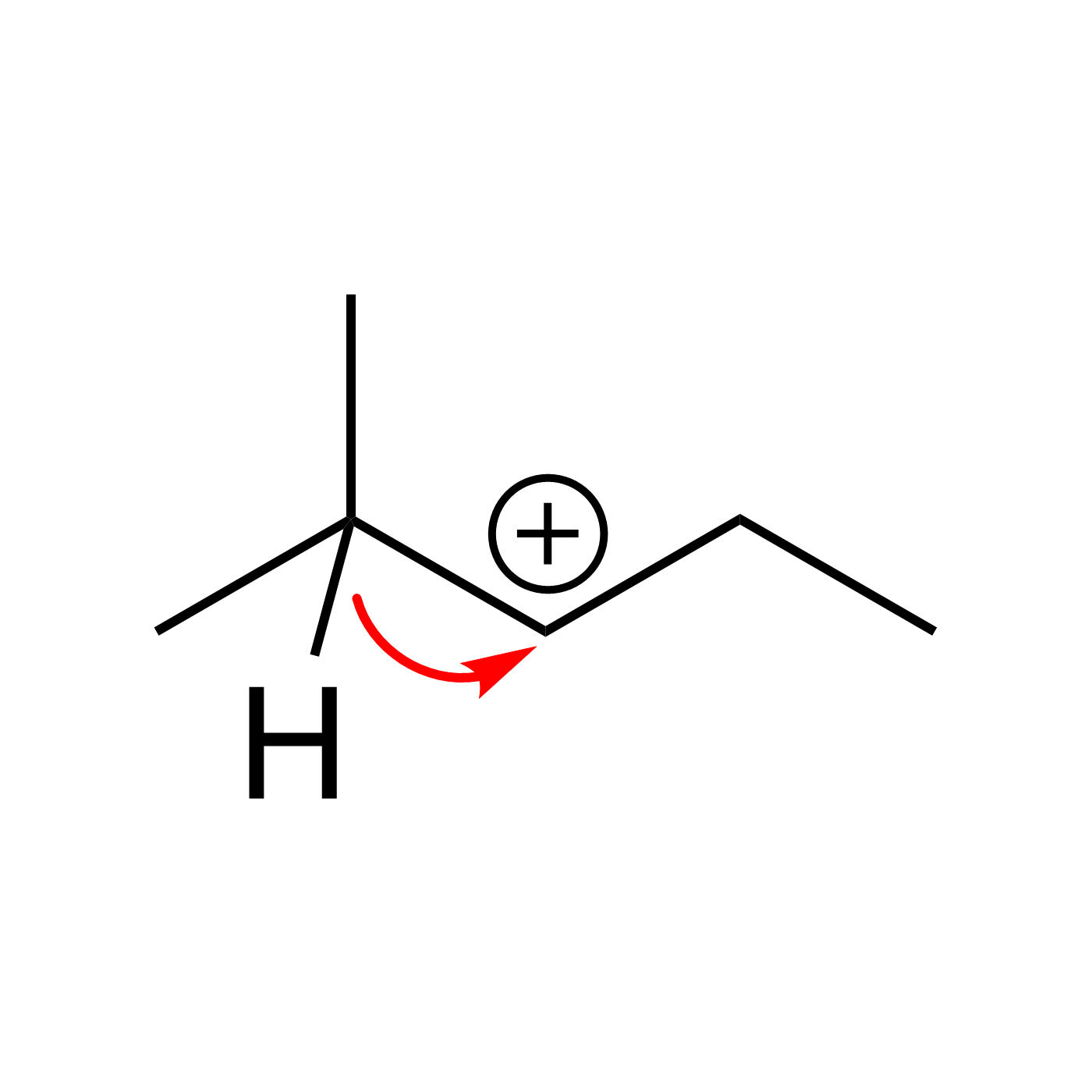
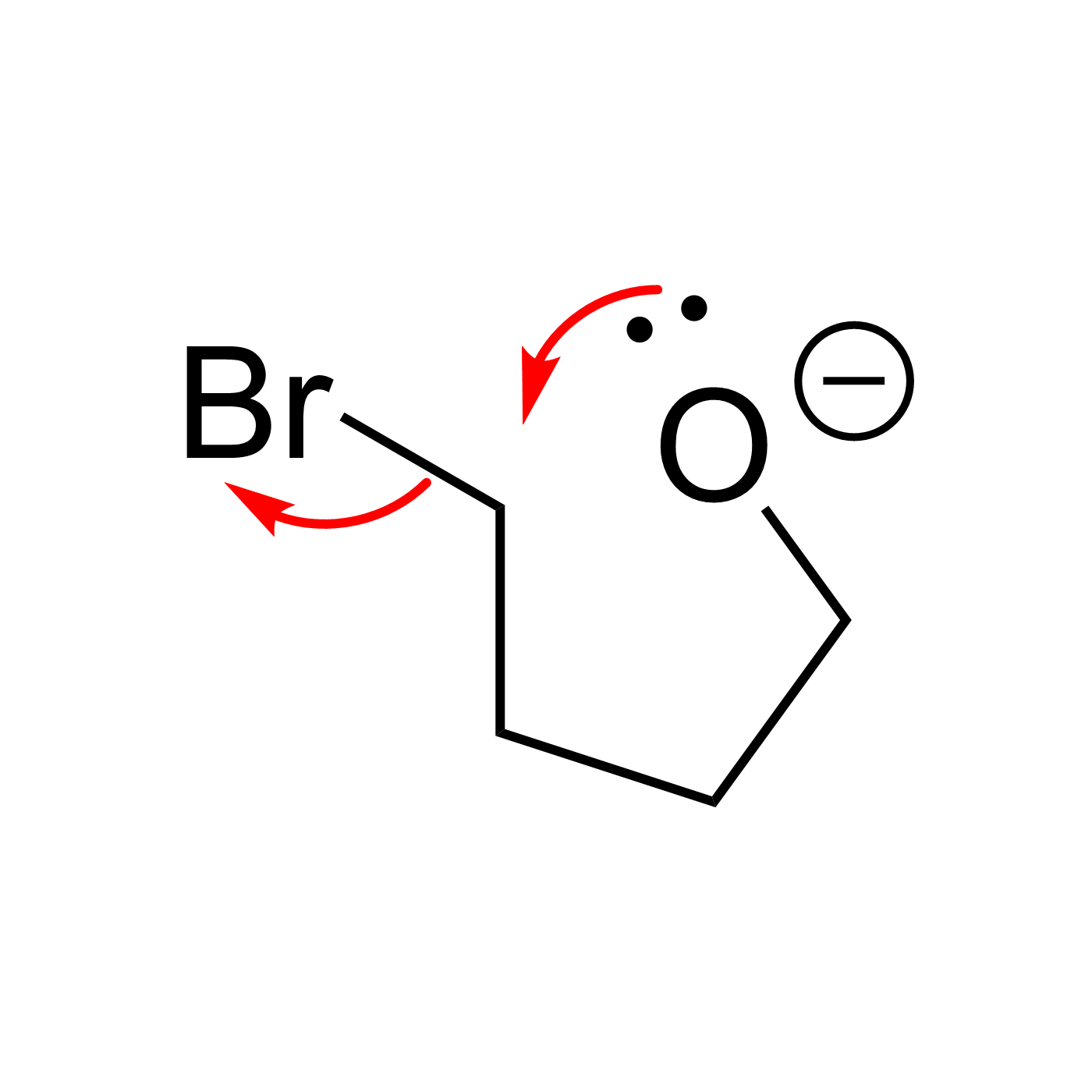
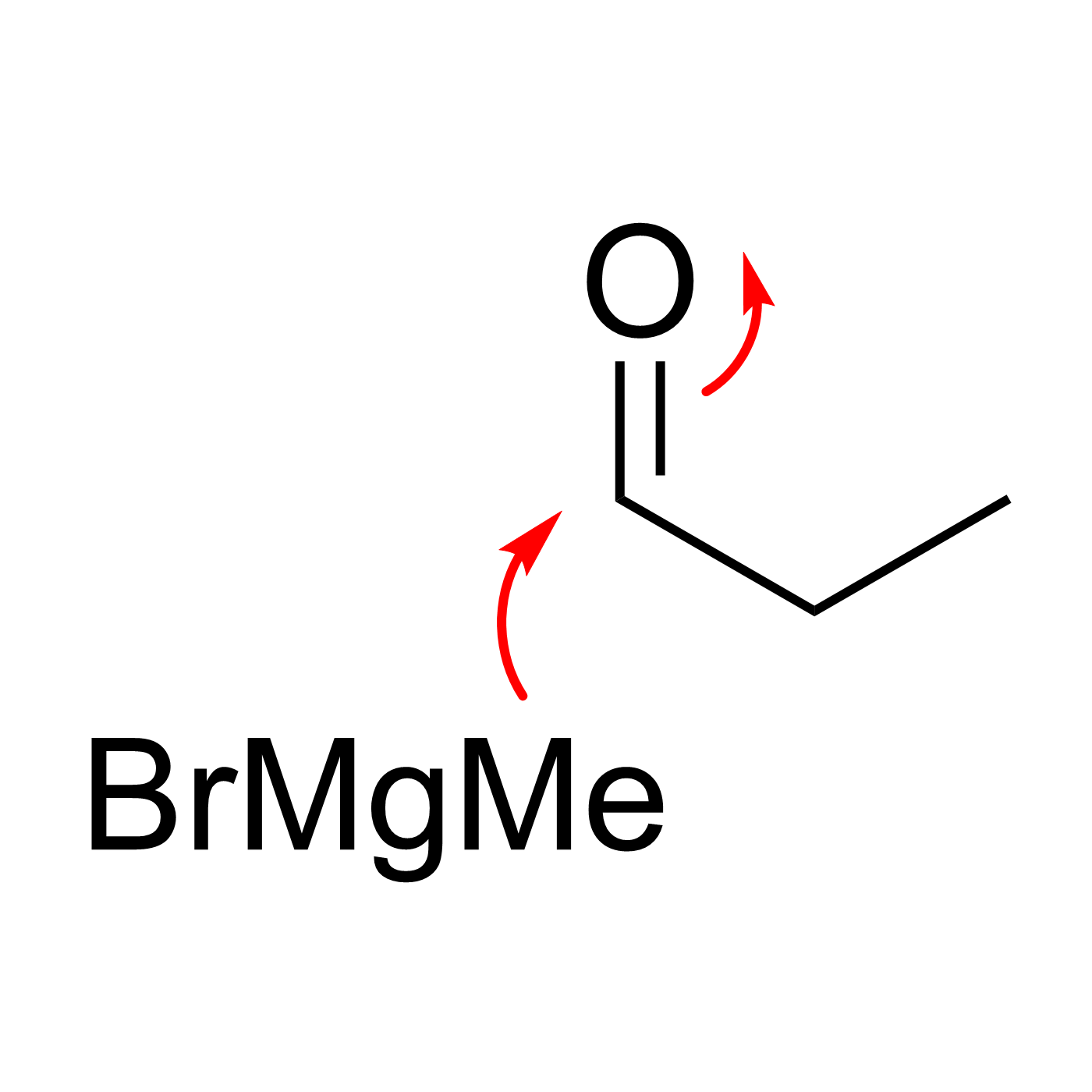
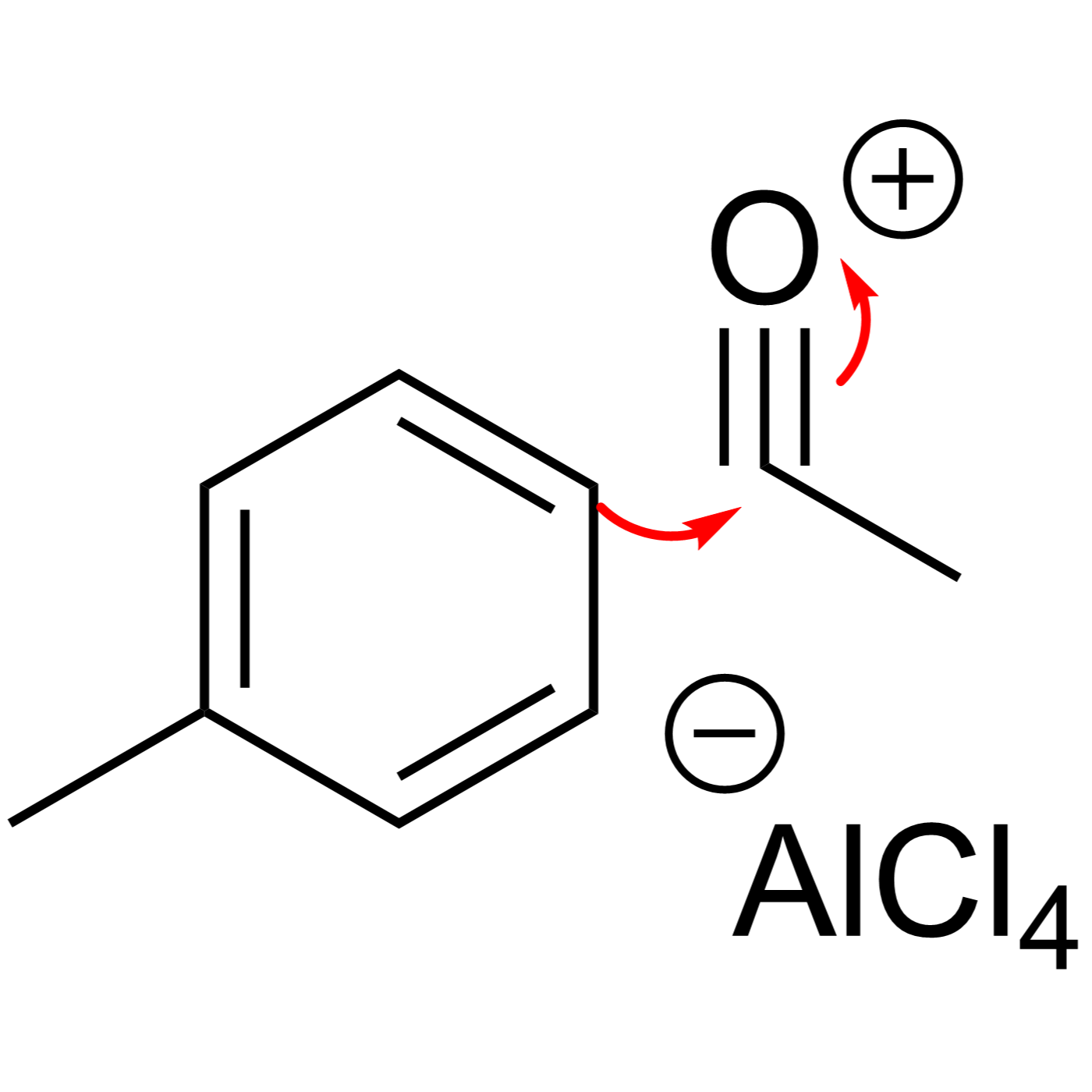
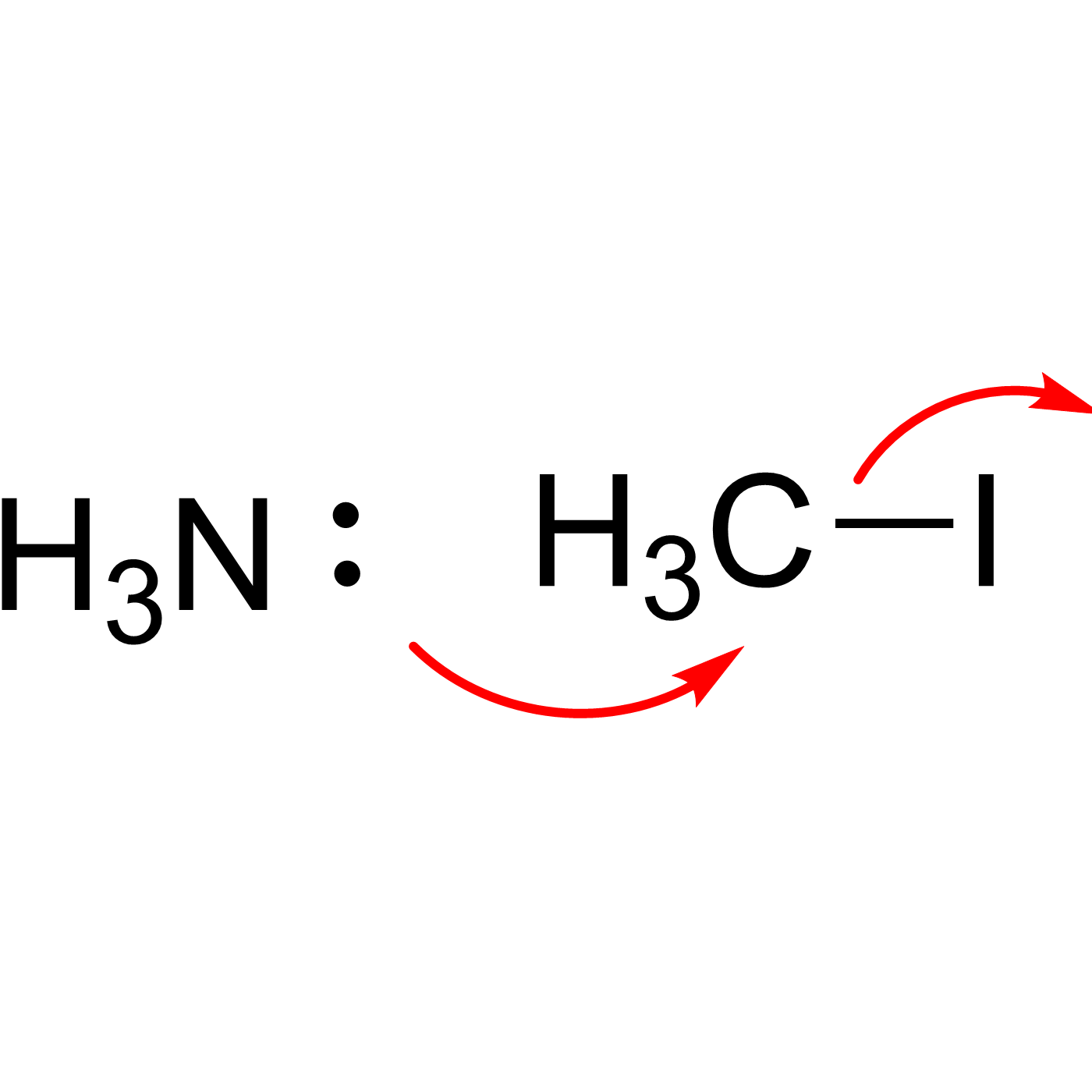
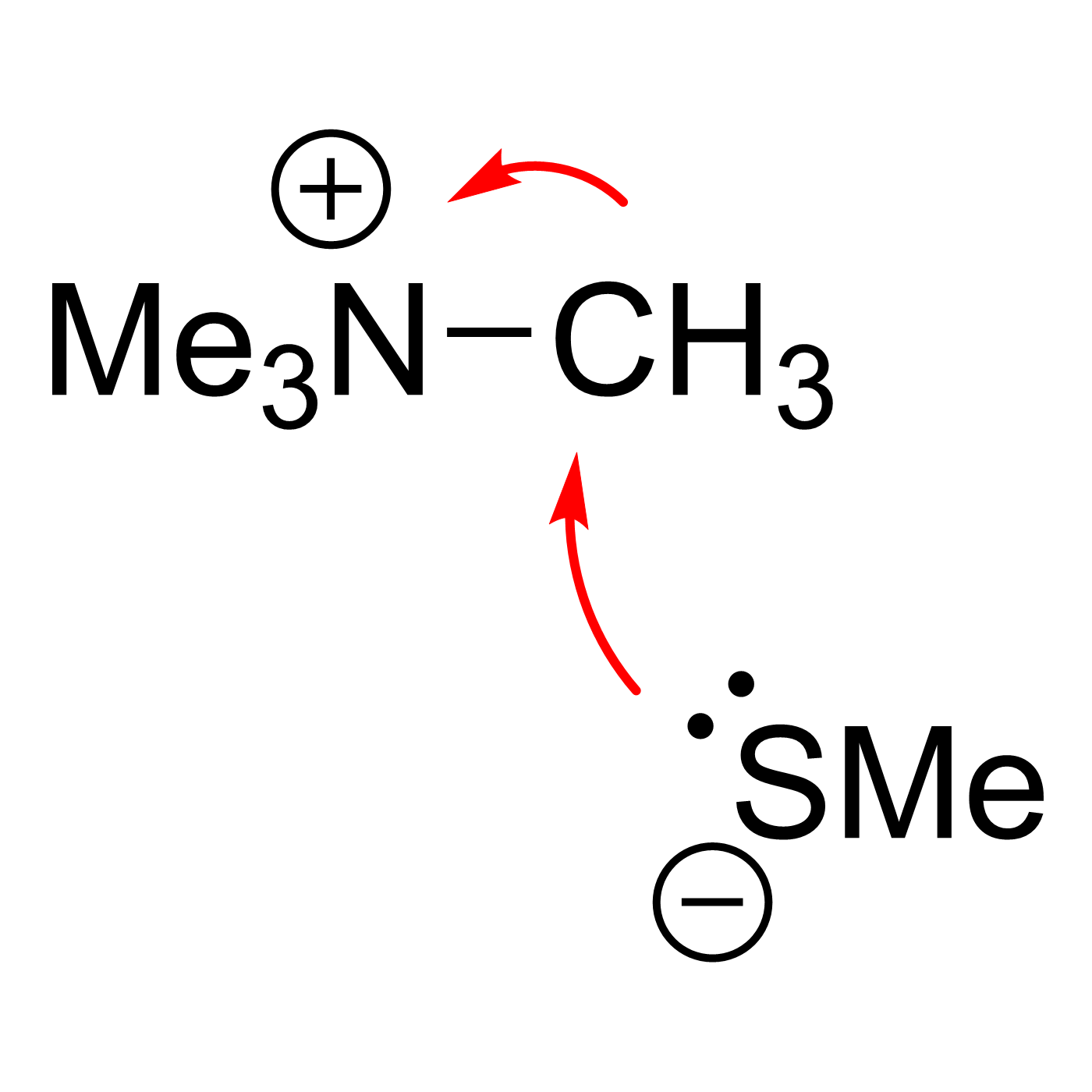
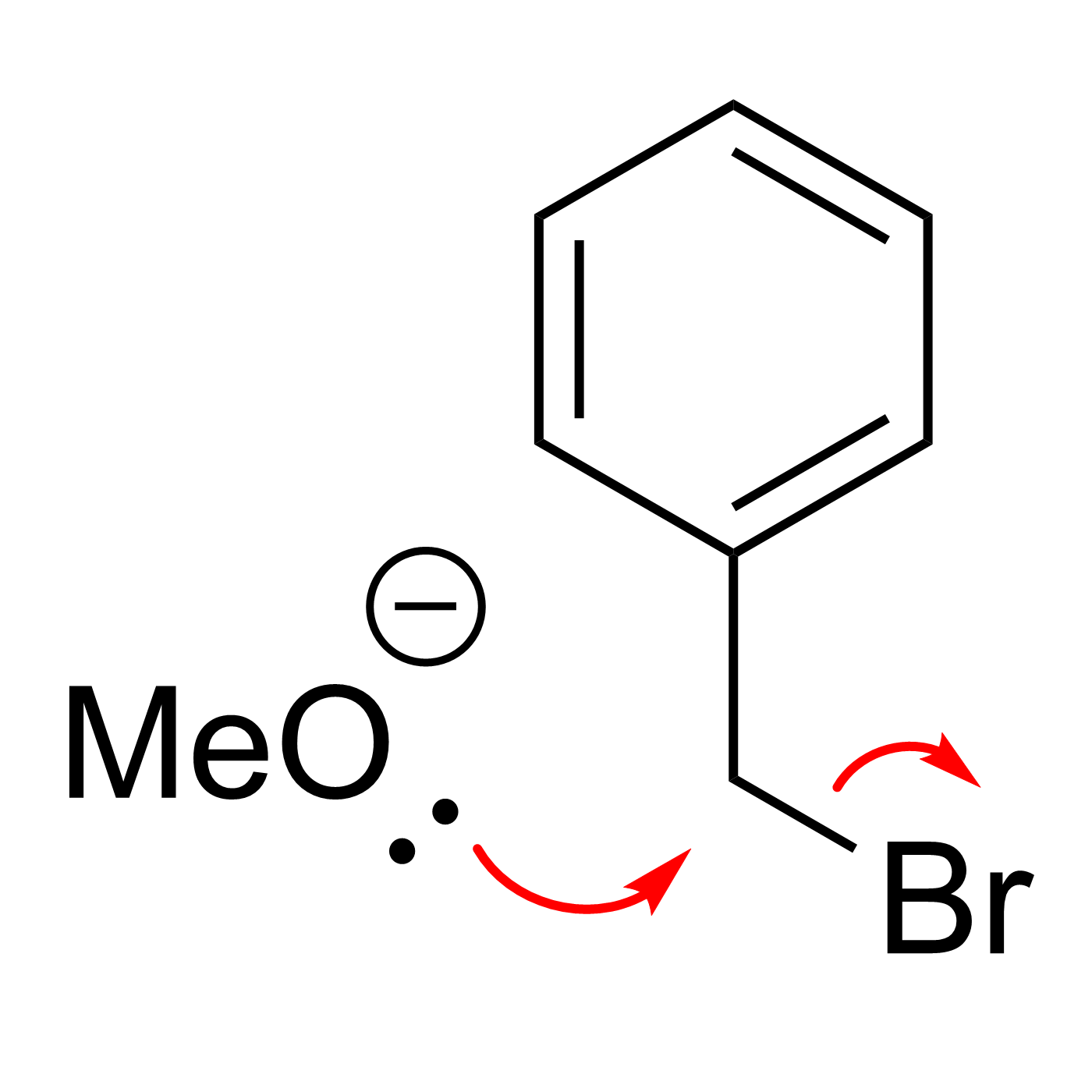
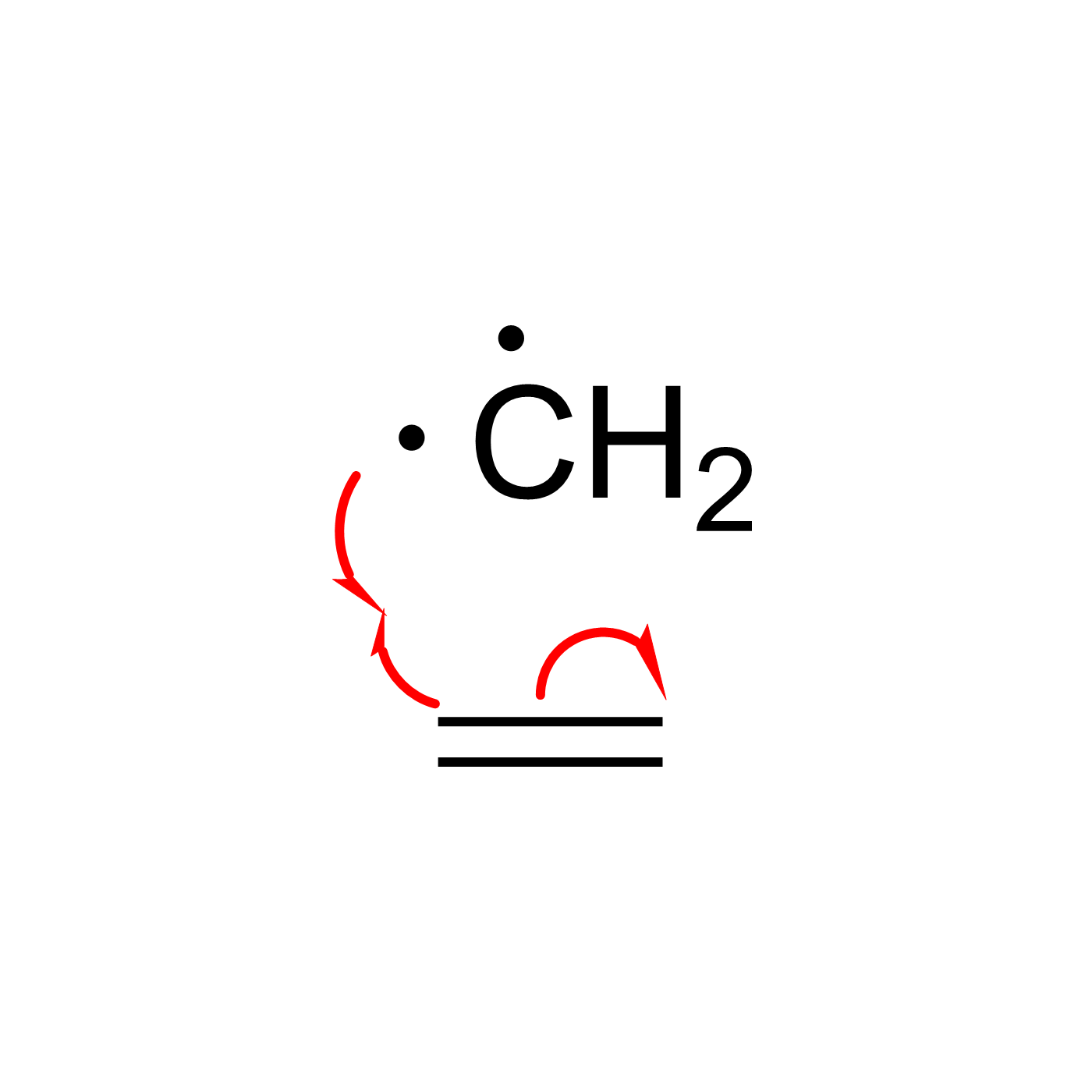
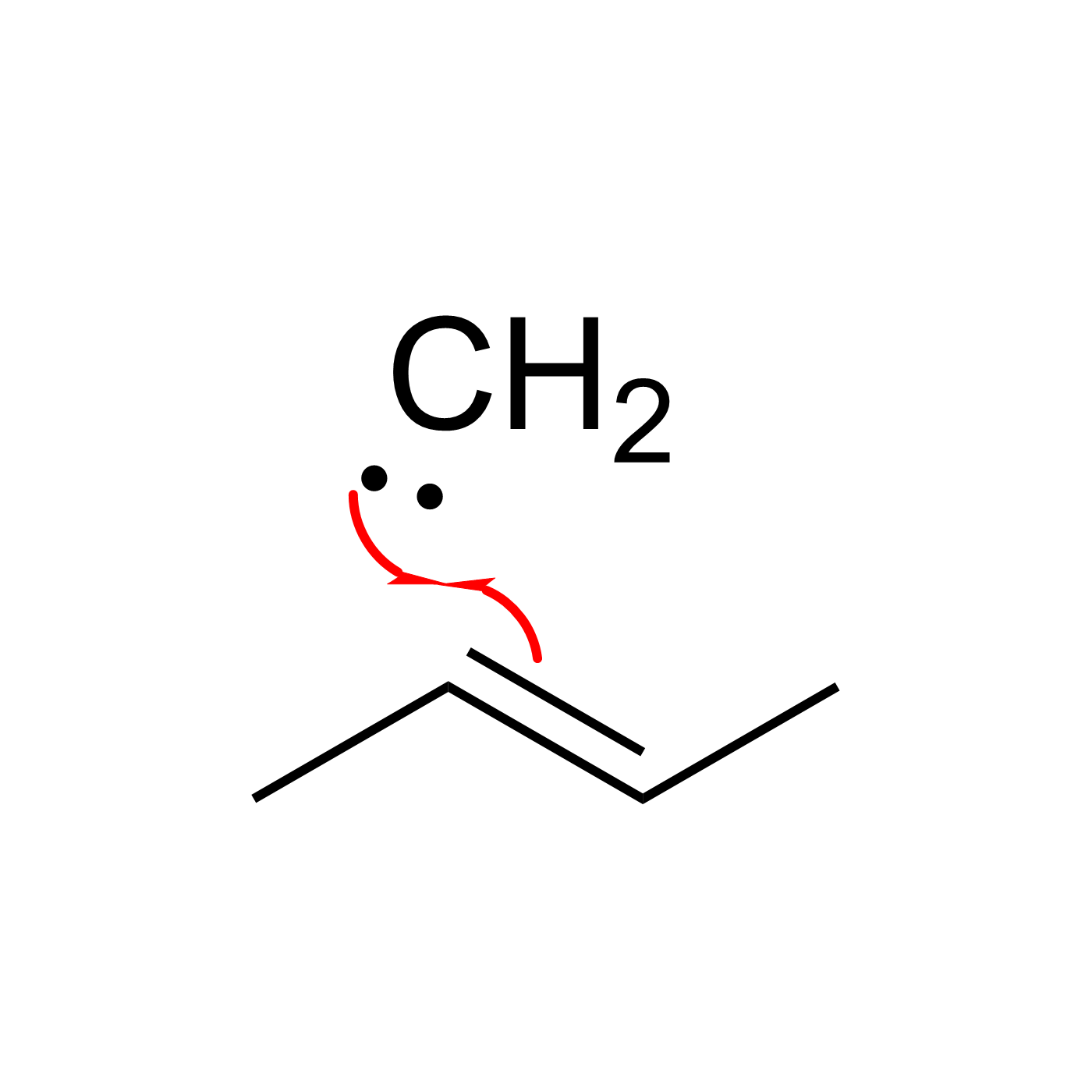
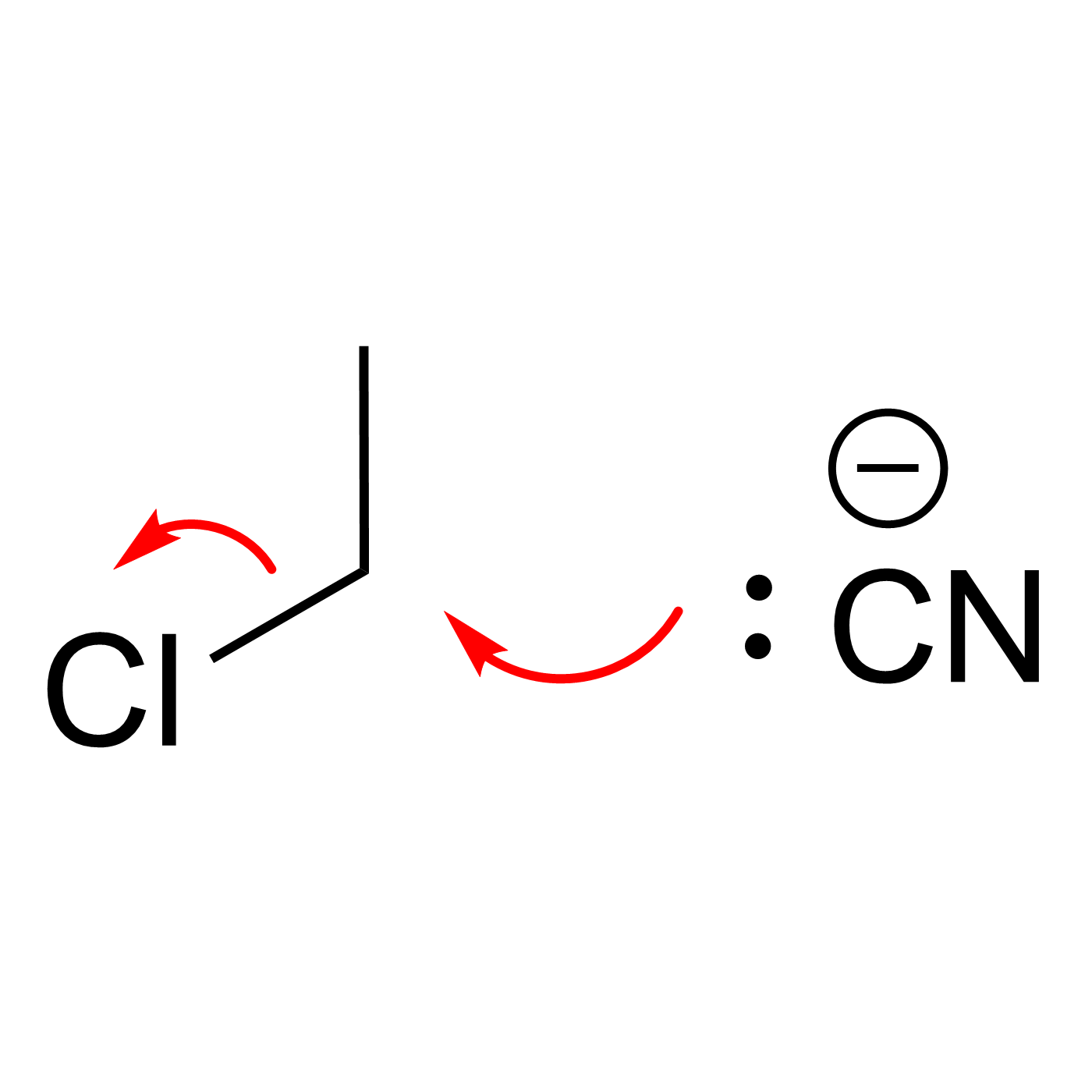
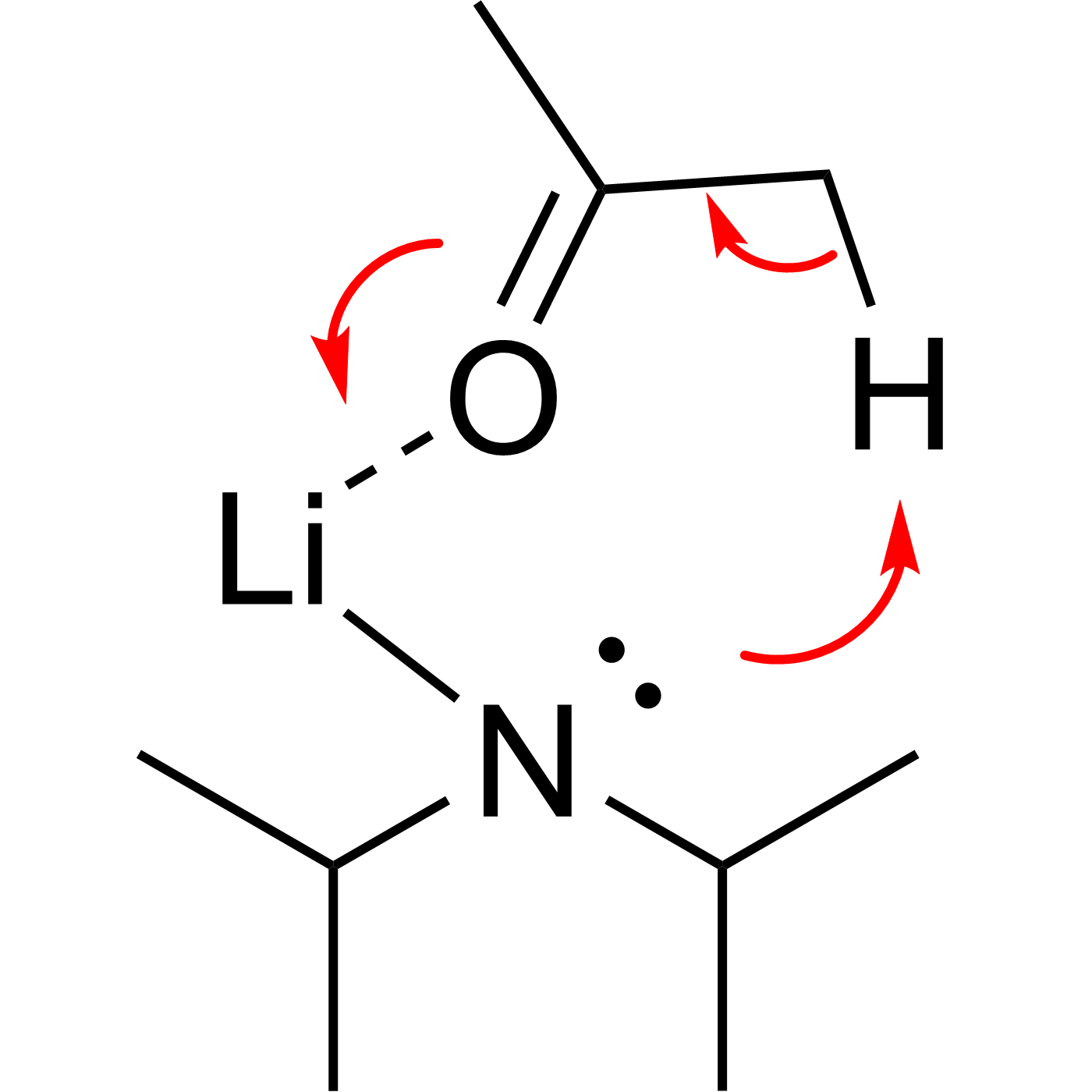
This website can be cited as:
Chen, S.; Fleming, S. A.; Ess, D. H. WebORA, 2021, http://webora.chem.byu.edu/.
Download the iPhone App by searching “iORA” on Apple App Store
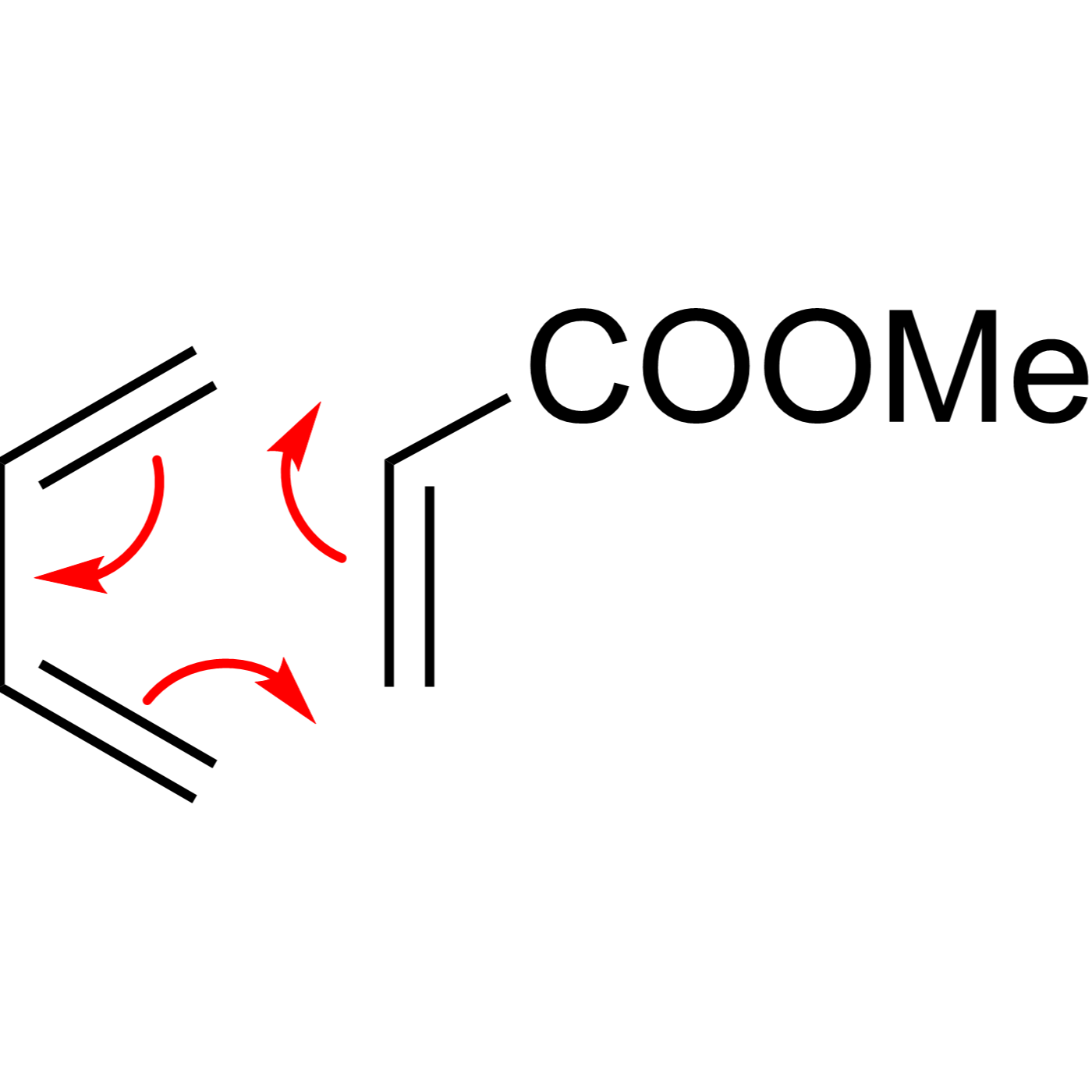
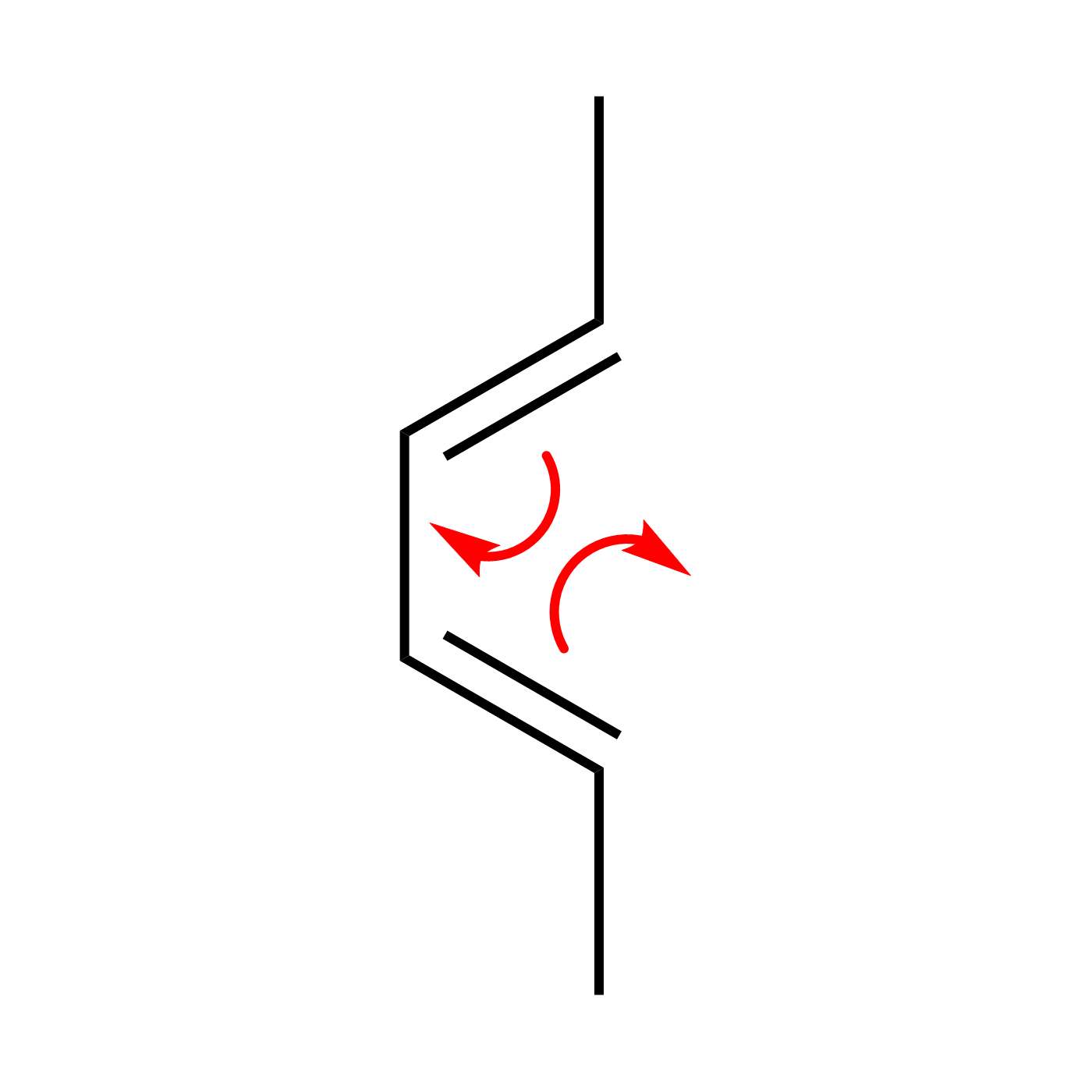
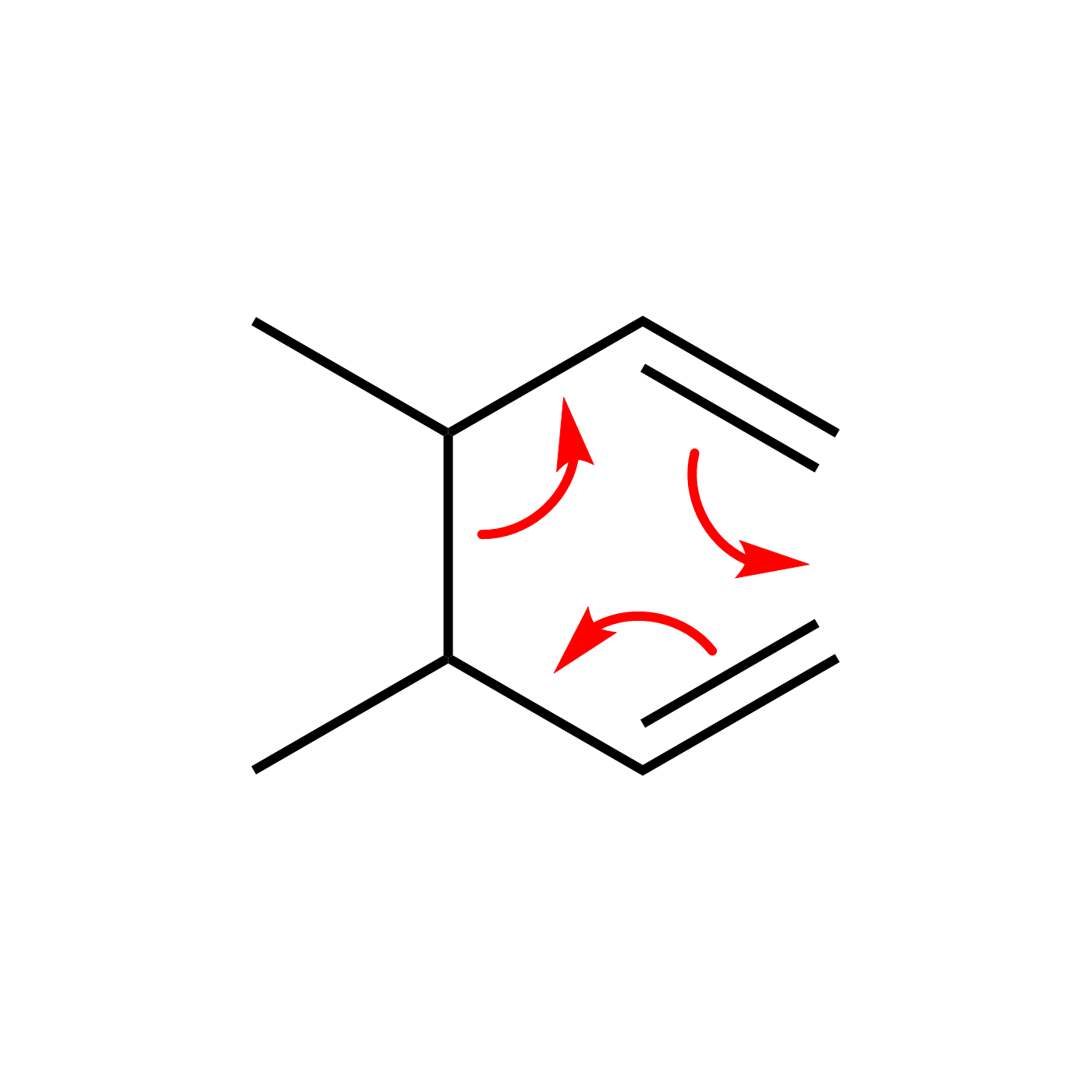
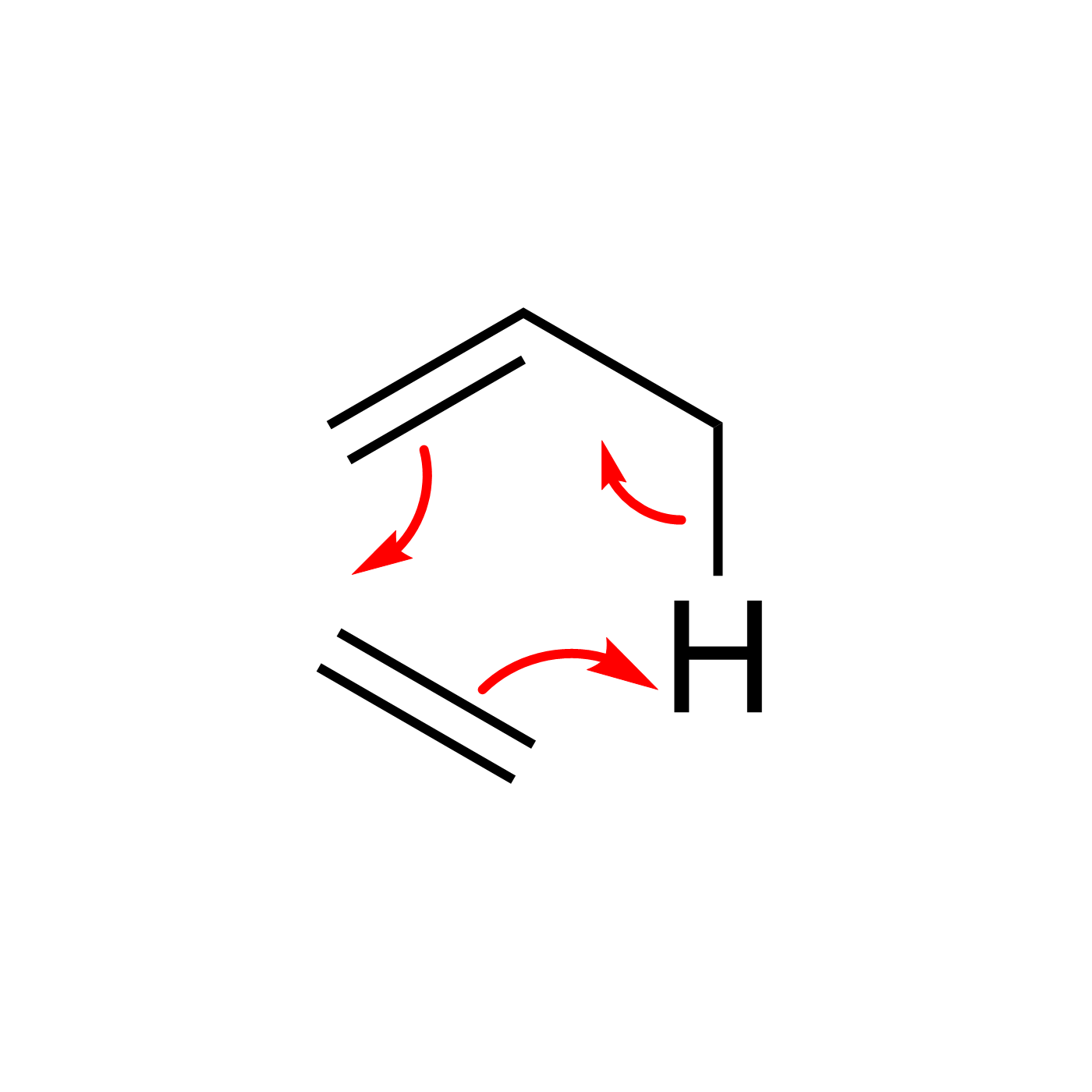
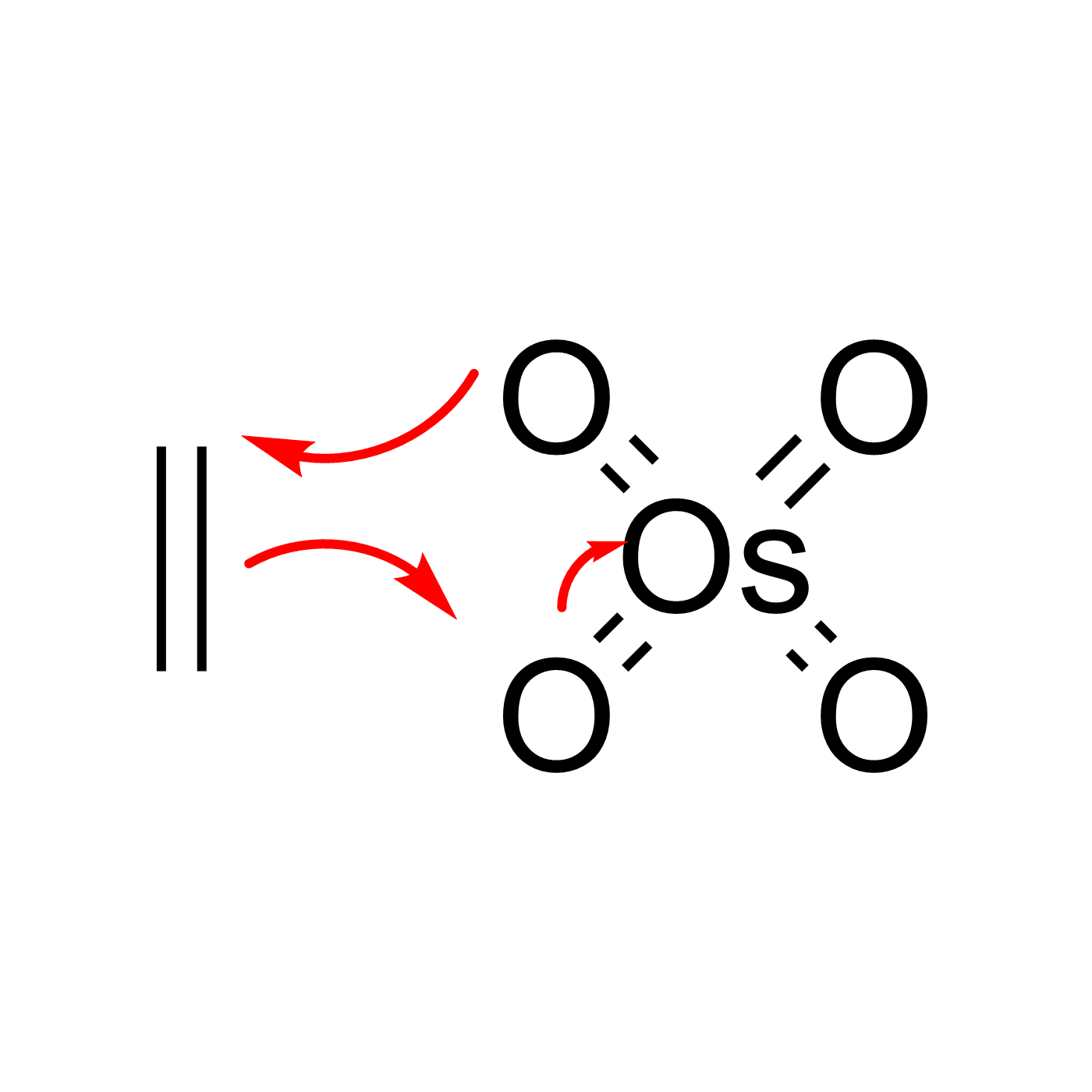
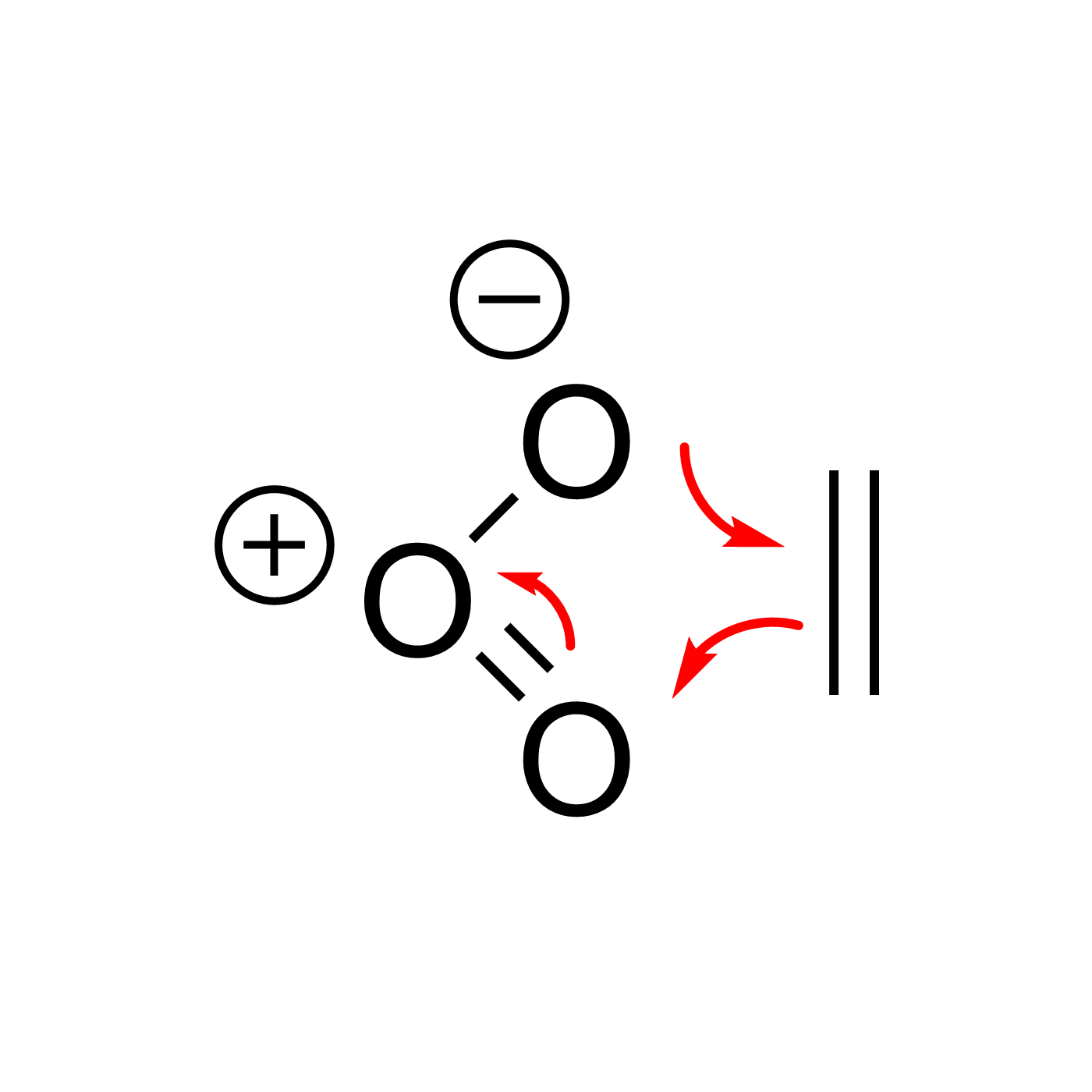
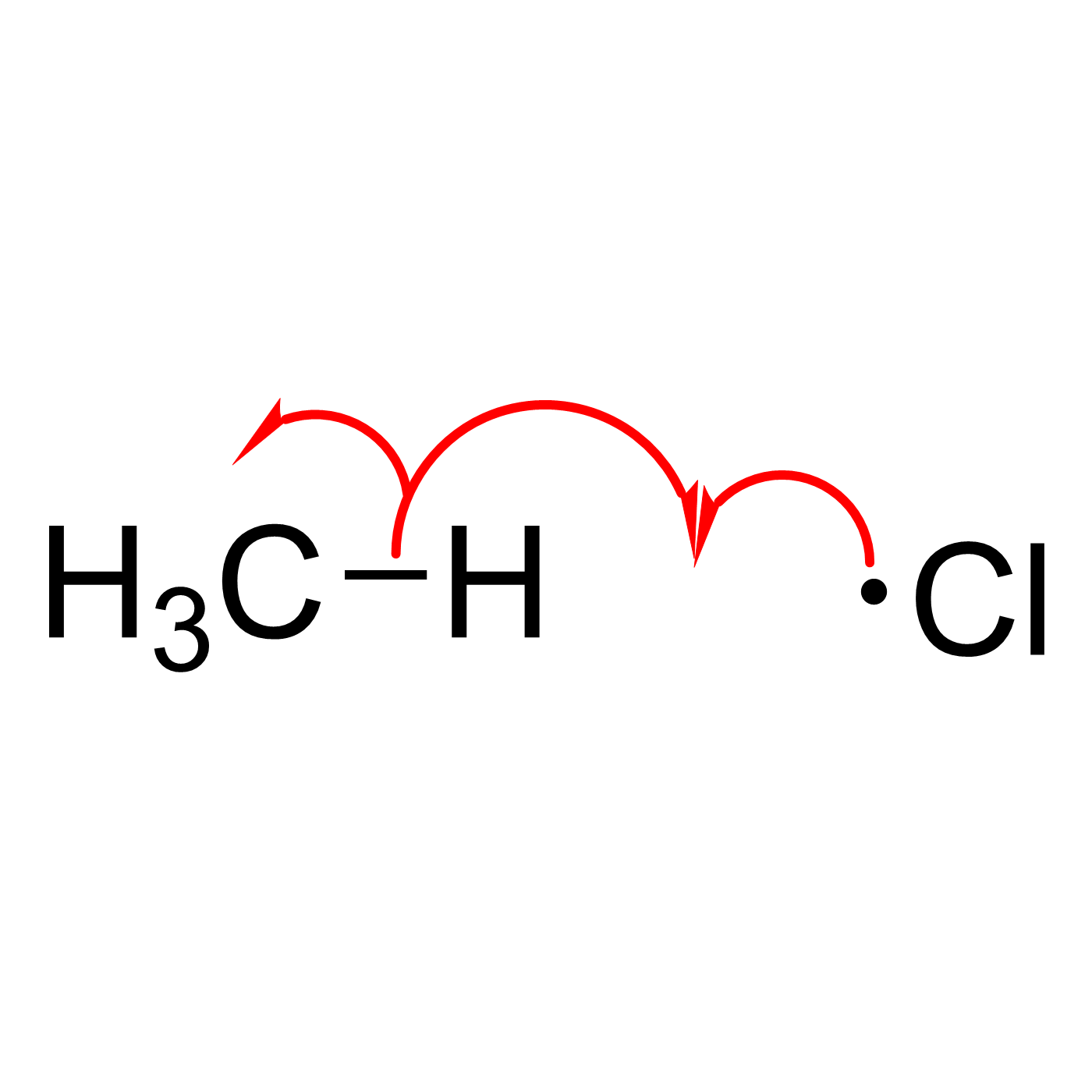
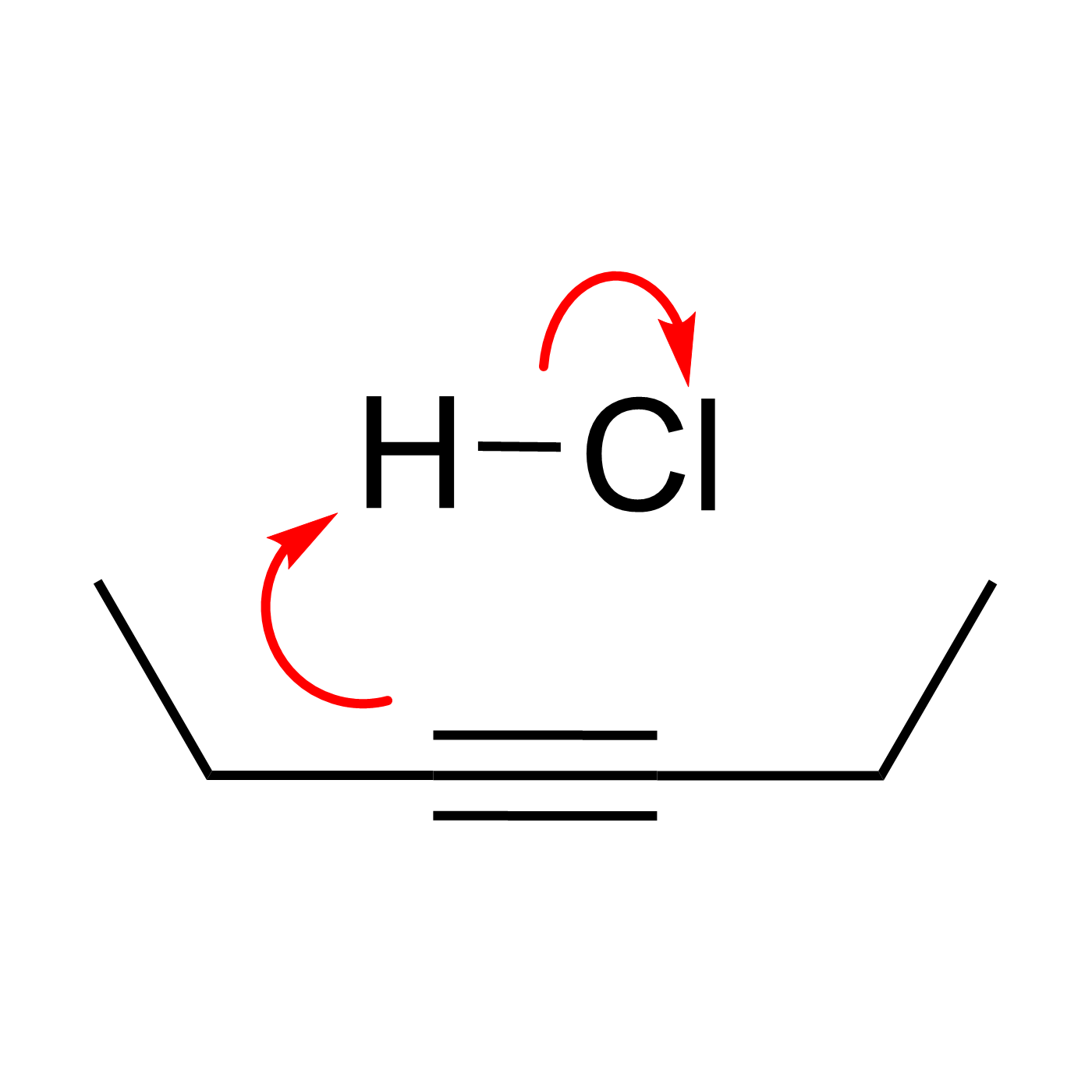
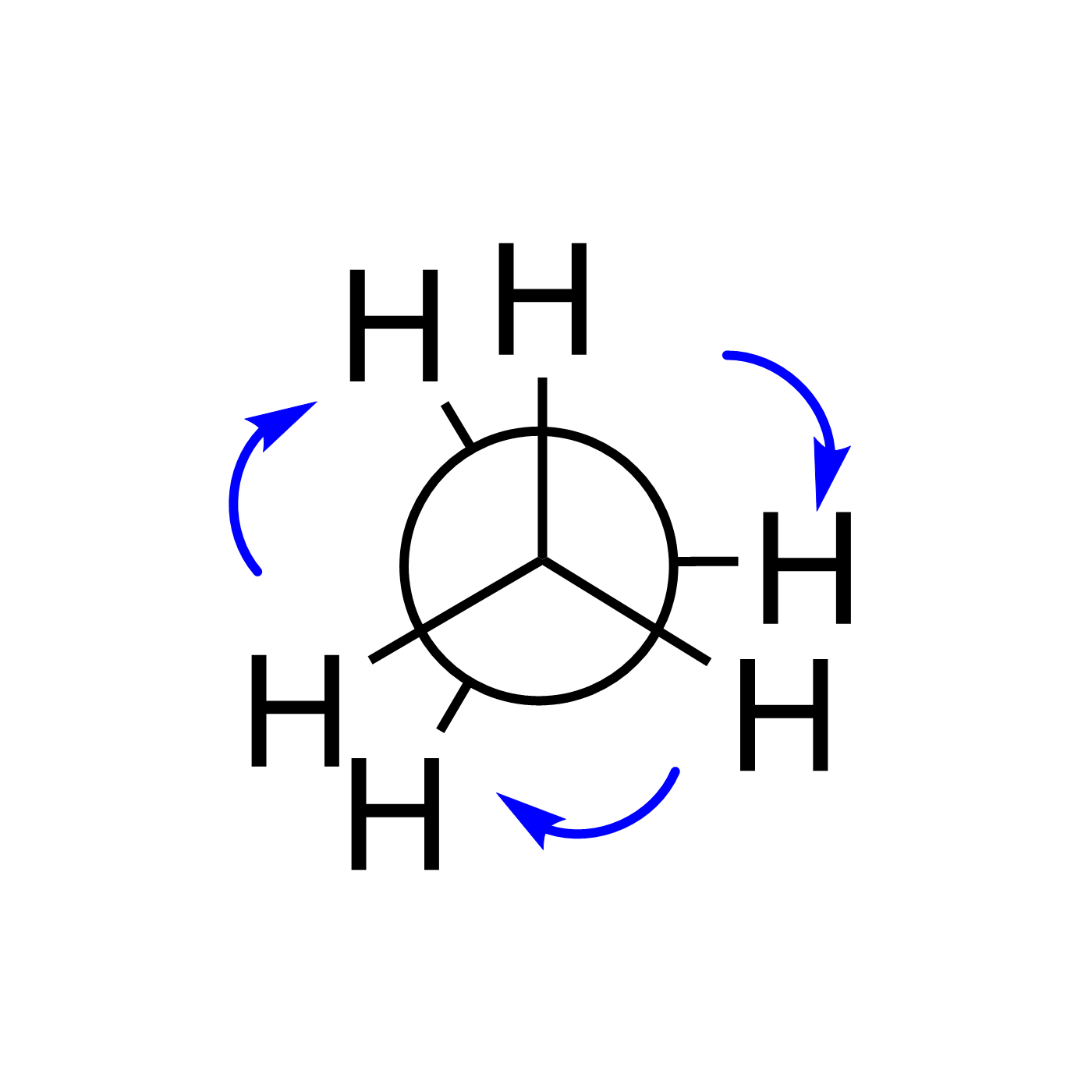
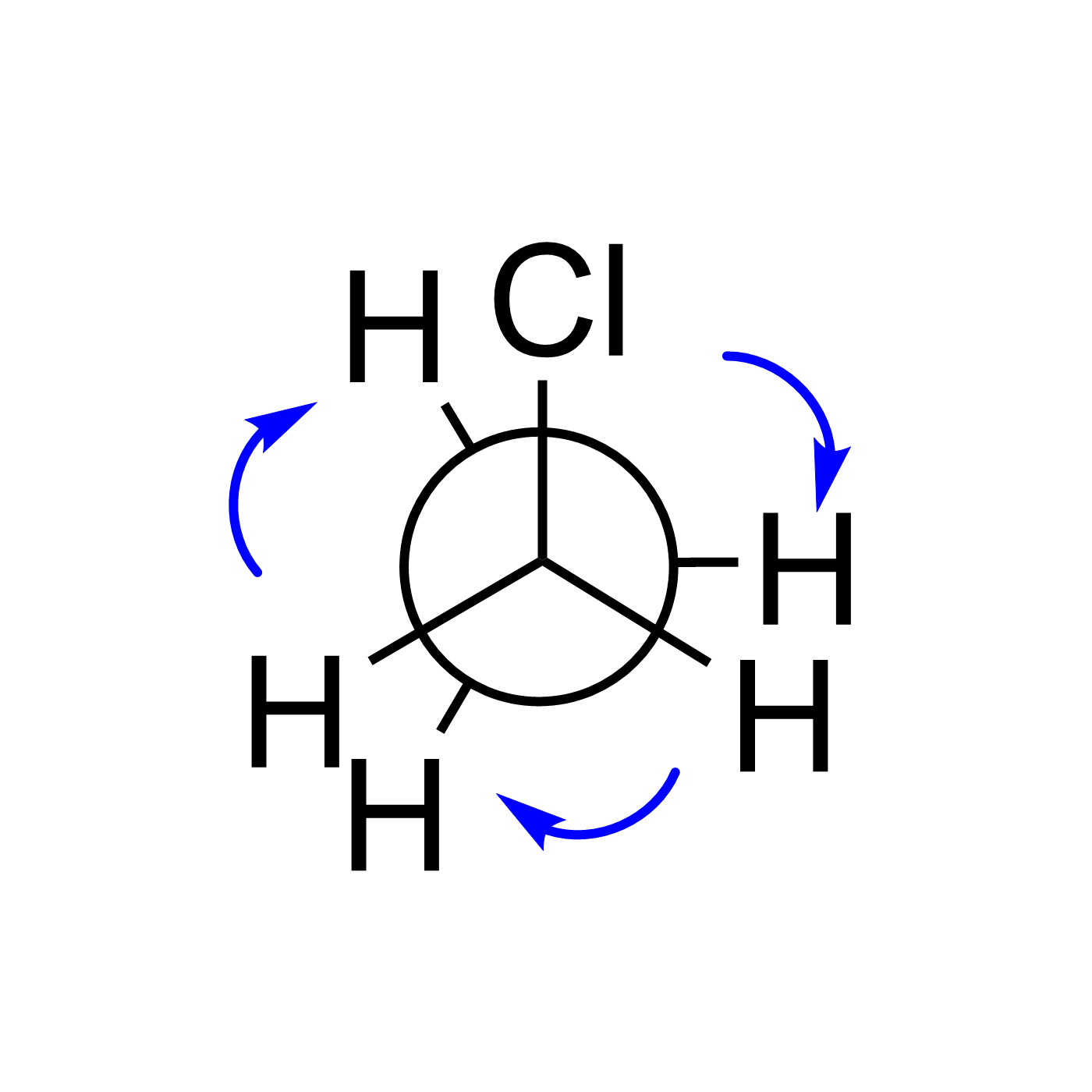
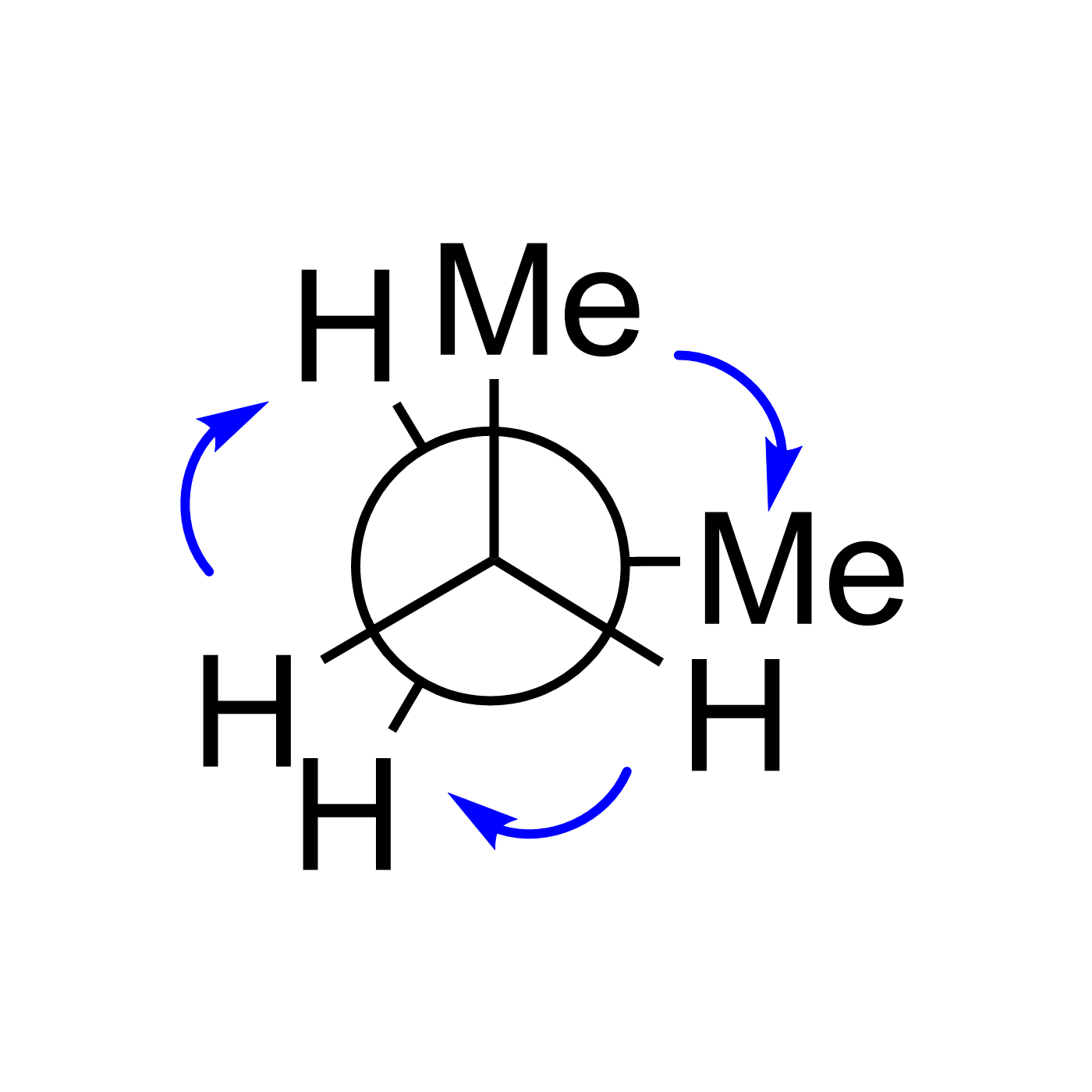
WebORA
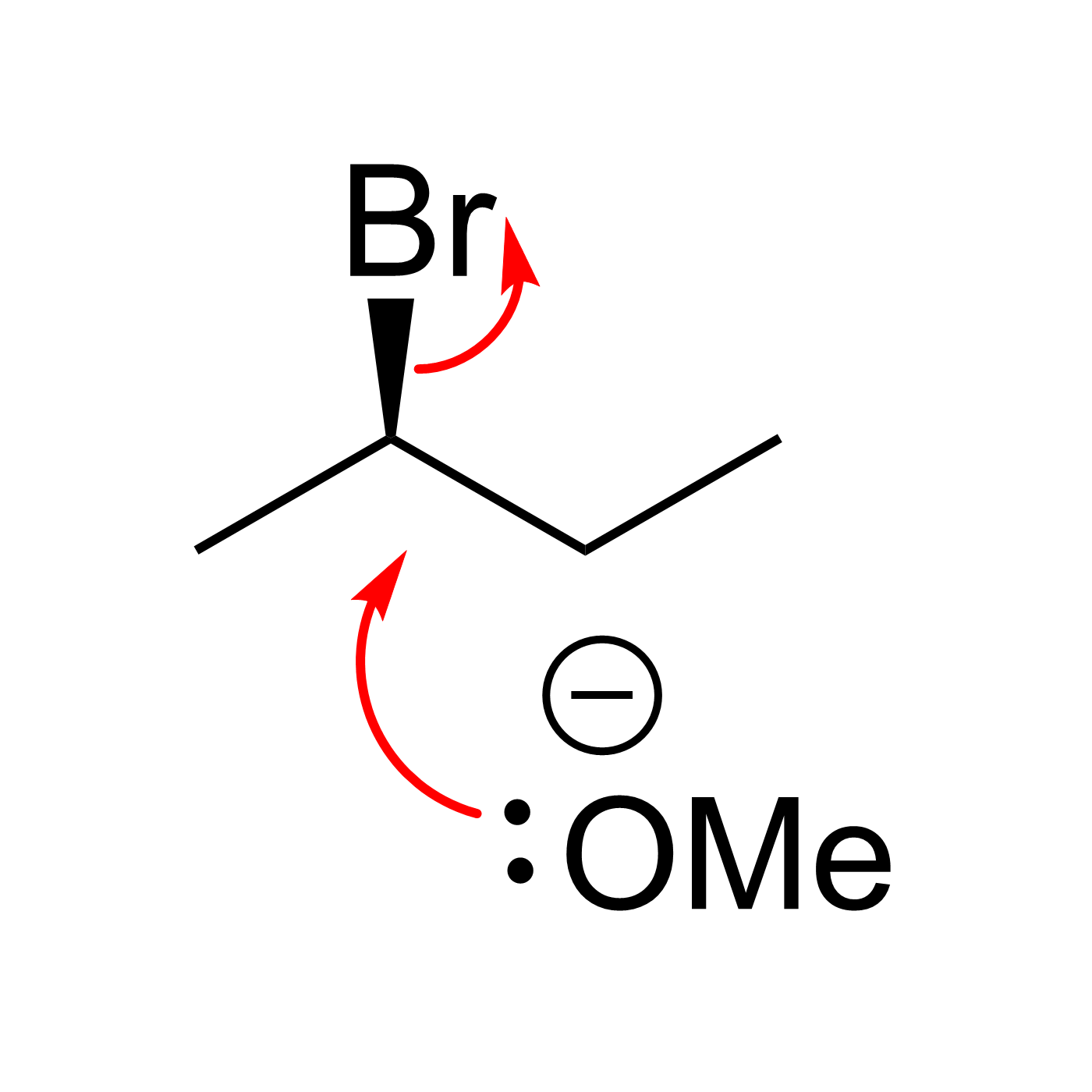
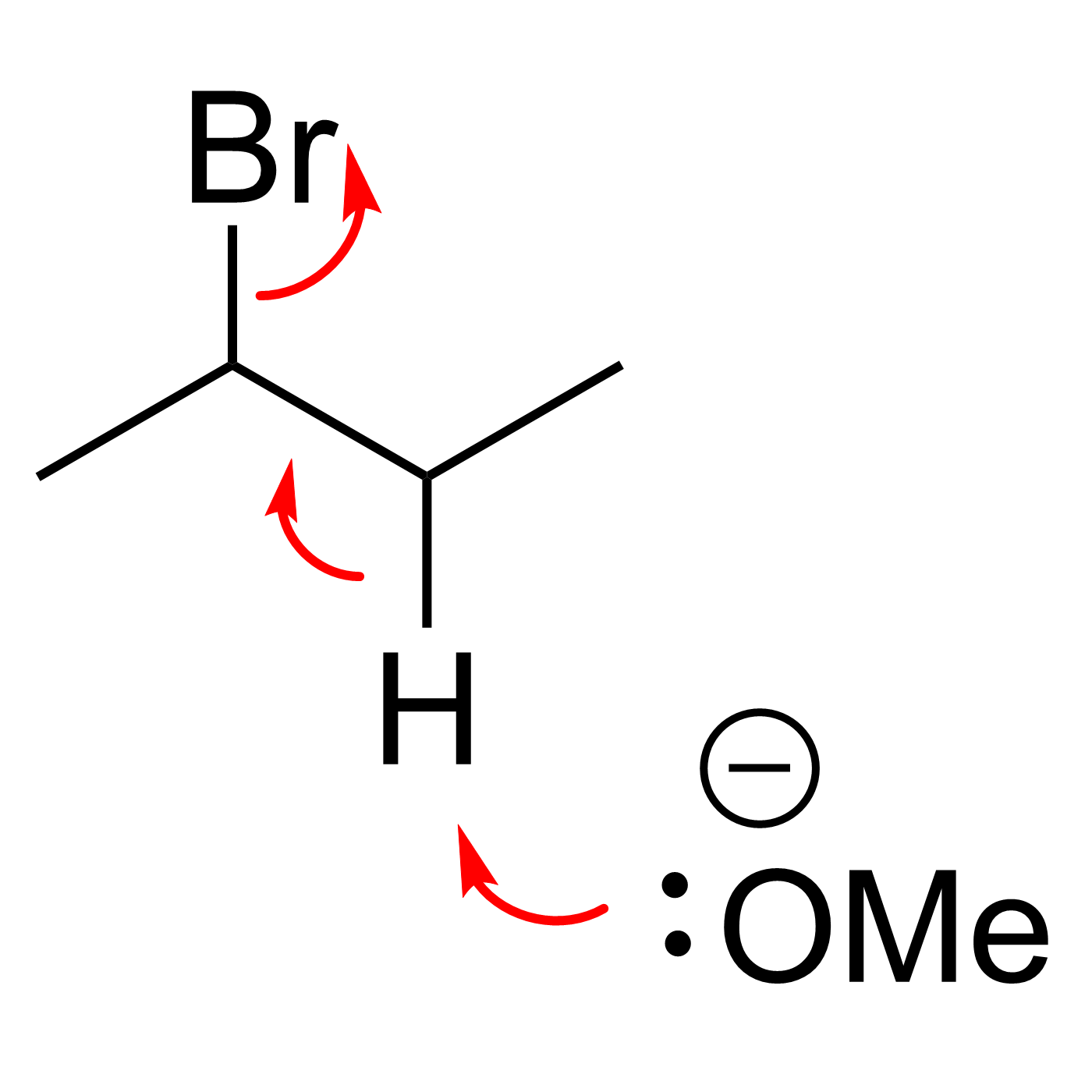
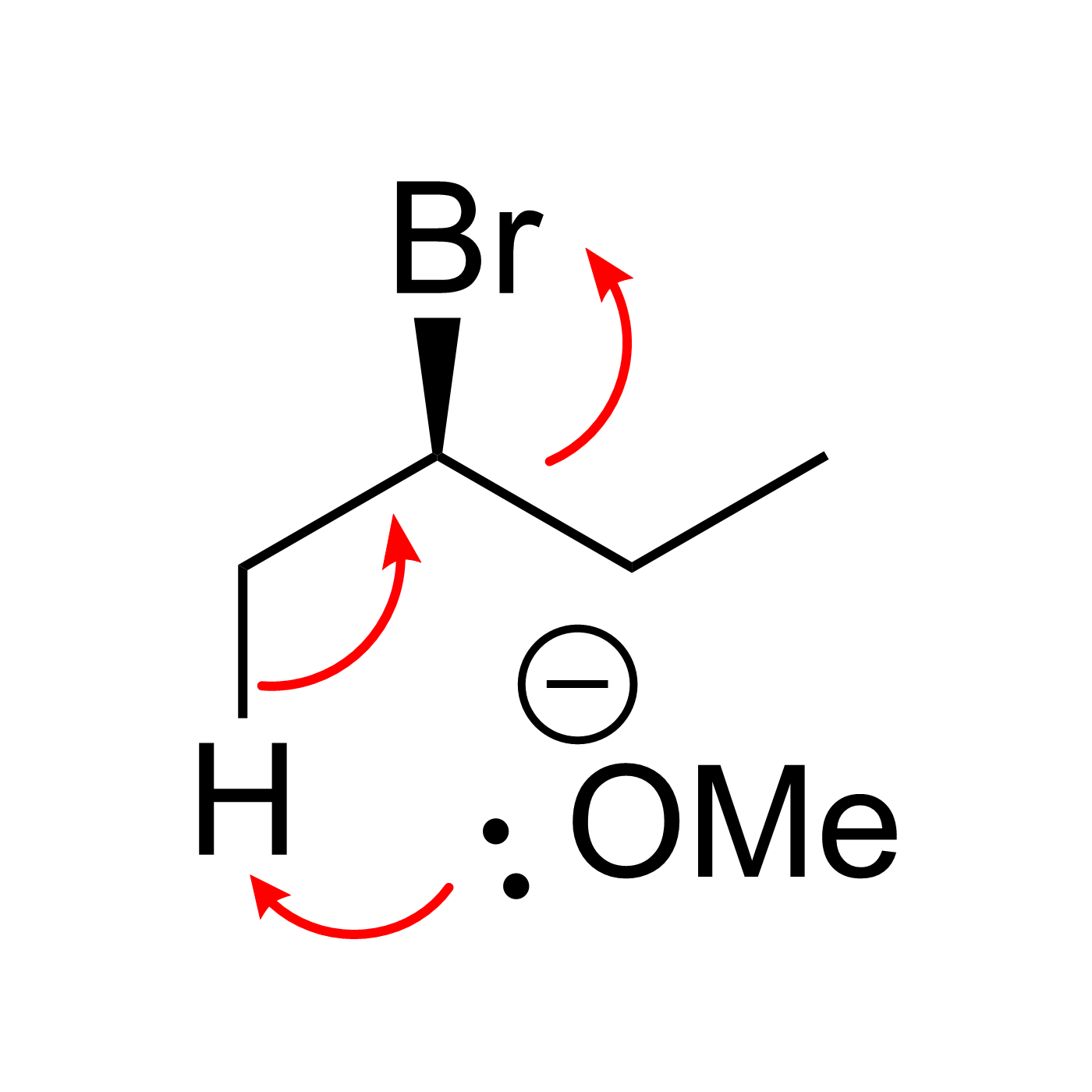
Organic Reaction Trajectories
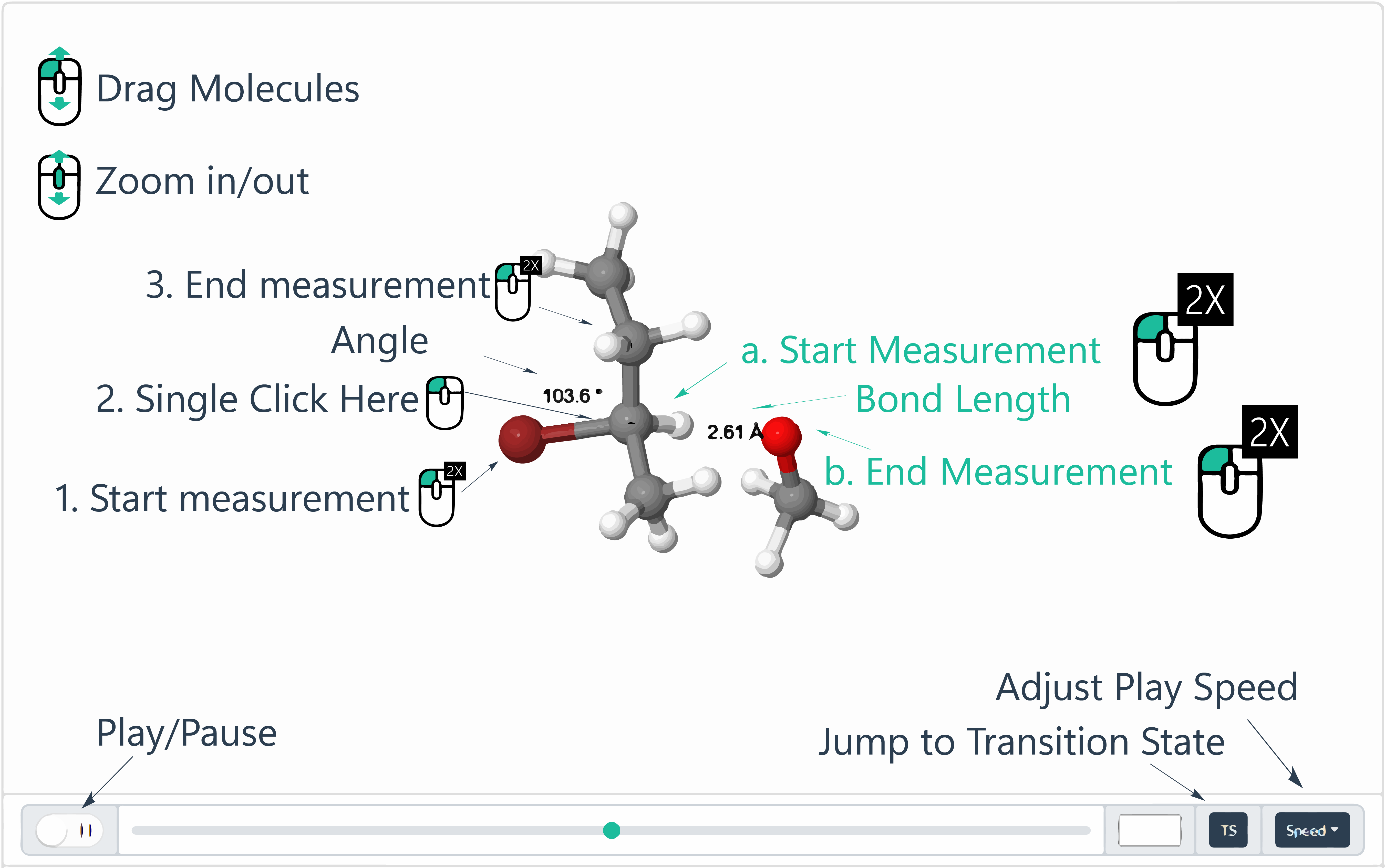
How to use?
(Click to Zoom in)